LY2334737Anticancer agent CAS# 892128-60-8 |
- SB 203580
Catalog No.:BCC3663
CAS No.:152121-47-6
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- PH-797804
Catalog No.:BCC3672
CAS No.:586379-66-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 892128-60-8 | SDF | Download SDF |
| PubChem ID | 11646777 | Appearance | Powder |
| Formula | C17H25F2N3O5 | M.Wt | 389.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (256.81 mM) *"≥" means soluble, but saturation unknown. | ||
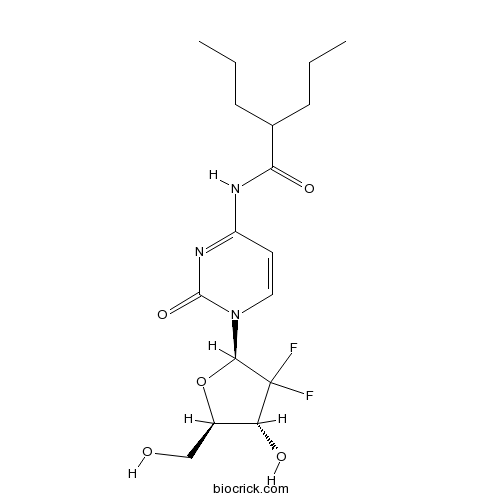
| Chemical Name | N-[1-[(2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-2-oxopyrimidin-4-yl]-2-propylpentanamide | ||
| SMILES | CCCC(CCC)C(=O)NC1=NC(=O)N(C=C1)C2C(C(C(O2)CO)O)(F)F | ||
| Standard InChIKey | MEOYFIHNRBNEPI-UXIGCNINSA-N | ||
| Standard InChI | InChI=1S/C17H25F2N3O5/c1-3-5-10(6-4-2)14(25)20-12-7-8-22(16(26)21-12)15-17(18,19)13(24)11(9-23)27-15/h7-8,10-11,13,15,23-24H,3-6,9H2,1-2H3,(H,20,21,25,26)/t11-,13-,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

LY2334737 Dilution Calculator

LY2334737 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5681 mL | 12.8406 mL | 25.6812 mL | 51.3624 mL | 64.203 mL |
| 5 mM | 0.5136 mL | 2.5681 mL | 5.1362 mL | 10.2725 mL | 12.8406 mL |
| 10 mM | 0.2568 mL | 1.2841 mL | 2.5681 mL | 5.1362 mL | 6.4203 mL |
| 50 mM | 0.0514 mL | 0.2568 mL | 0.5136 mL | 1.0272 mL | 1.2841 mL |
| 100 mM | 0.0257 mL | 0.1284 mL | 0.2568 mL | 0.5136 mL | 0.642 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LY2334737 is an oral prodrug of gemcitabine, the clinically efficacious anticancer agent [1].
LY2334737 has been shown the hydrolytic activity by carboxylesterase 2 (CES2). The Michaelis-Menten kinetic parameters are exhibited to be : Km of 43μM and Vmax of 40nmol/min/mg protein. In addition, LY2334737 has been found to be cytotoxic to cells in the NCl-60 panel with the EC50 values of 0.512±0.016μM, 0.402±0.018μM and 0.034±0.002μM for PC-GFP alone, HCT-116 mock transfectant and HCT-116 CES2 transfectant cell lines, respectively. Apart from these, the study result has been noted that parental SK-OV-3 cells were approximately 4-fold more sensitive to LY2334737 cytotoxicity than the SK-OV-3 knockdown with the EC50 values of 1.1μM and 4.5μM, respectively [1].
References:
[1] Pratt SE1, Durland-Busbice S, Shepard RL, Heinz-Taheny K, Iversen PW, Dantzig AH. Human carboxylesterase-2 hydrolyzes the prodrug of gemcitabine (LY2334737) and confers prodrug sensitivity to cancer cells. Clin Cancer Res. 2013 Mar 1;19(5):1159-68.
- Mulberrofuran H
Catalog No.:BCN3371
CAS No.:89199-99-5
- Fraxiresinol 1-O-glucoside
Catalog No.:BCN4439
CAS No.:89199-94-0
- Efaroxan hydrochloride
Catalog No.:BCC6797
CAS No.:89197-00-2
- 15,16-Dihydrotanshindiol C
Catalog No.:BCN3214
CAS No.:891854-96-9
- 1,2-Didehydrocryptotanshinone
Catalog No.:BCN3122
CAS No.:891854-92-5
- 15,16-Dihydrotanshindiol B
Catalog No.:BCN3213
CAS No.:891854-86-7
- SCH900776 S-isomer
Catalog No.:BCC1936
CAS No.:891494-64-7
- MK-8776 (SCH-900776)
Catalog No.:BCC3817
CAS No.:891494-63-6
- Betulin caffeate
Catalog No.:BCN4438
CAS No.:89130-86-9
- Odoratisol A
Catalog No.:BCN7813
CAS No.:891182-93-7
- 4-O-Demethylisokadsurenin D
Catalog No.:BCN6650
CAS No.:89104-59-6
- BAMB-4
Catalog No.:BCC5428
CAS No.:891025-25-5
- Manidipine
Catalog No.:BCC4404
CAS No.:89226-50-6
- Manidipine 2HCl
Catalog No.:BCC4405
CAS No.:89226-75-5
- AZD 3988
Catalog No.:BCC5621
CAS No.:892489-52-0
- 2-(Chloromethyl)-4-(4-nitrophenyl)-1,3-thiazole
Catalog No.:BCC8372
CAS No.:89250-26-0
- MF63
Catalog No.:BCC1744
CAS No.:892549-43-8
- 2,4-Dihydroxy-3-nitropyridine
Catalog No.:BCC8499
CAS No.:89282-12-2
- 3,4-O,O-Methylene-(+)-catechin
Catalog No.:BCN7962
CAS No.:89329-14-6
- ICI 174,864
Catalog No.:BCC5675
CAS No.:89352-67-0
- Chiisanoside
Catalog No.:BCN2712
CAS No.:89354-01-8
- Riligustilide
Catalog No.:BCC9136
CAS No.:89354-45-0
- PF 945863
Catalog No.:BCC6172
CAS No.:893556-85-9
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
Phase 1 dose escalation and pharmacokinetic evaluation of oral gemcitabine prodrug (LY2334737) in combination with docetaxel in patients with advanced solid tumors.[Pubmed:24744161]
Cancer Chemother Pharmacol. 2014 Jun;73(6):1205-15.
PURPOSE: This Phase 1 study aimed to determine the recommended Phase 2 dose of LY2334737, an oral gemcitabine prodrug, when combined with standard dose docetaxel treatment in patients with advanced solid tumors. Pharmacokinetics (PK) and antitumor activity were additionally evaluated. METHODS: Patients with advanced/metastatic solid tumors received escalating doses of LY2334737 once daily (QD) for 14 days, followed by a 7-day drug-free period. Docetaxel was given at 75 mg/m(2) every 3 weeks (q3w). Cycles were repeated until progressive disease (PD) or unacceptable toxicity. RESULTS: Of 22 patients recruited, all Caucasian, 7 received an LY2334737 dose of 10 mg/day, 10 received 20 mg/day, 5 received 30 mg/day. Nineteen patients discontinued due to PD, 2 due to adverse events, 1 due to investigator decision. Dose-limiting toxicities: 2x febrile neutropenia (G3), 2x fatigue (1x G2, 1x G3), 1x neutropenia (G4). The maximum tolerated dose (MTD) was identified to be 10 mg/day. Two patients achieved partial response, 10 patients stable disease. Enrollment was stopped after unexpected hepatic toxicities were observed with LY2334737 QD for 14 days per cycle in another study of Japanese patients. PK data were consistent with the first-in-man study of LY2334737 and did not reveal any drug-drug interaction between LY2334737 and docetaxel. CONCLUSIONS: Combination of LY2334737 at doses up to 30 mg/day QD for 14 days per cycle with docetaxel 75 mg/m(2) q3w resulted in an undesirable toxicity profile and a low MTD of 10 mg/day. Alternative treatment schedules of LY2334737 should be explored.
High sensitive assay employing column switching chromatography to enable simultaneous quantification of an amide prodrug of gemcitabine (LY2334737), gemcitabine, and its metabolite dFdU in human plasma by LC-MS/MS.[Pubmed:23831704]
J Chromatogr B Analyt Technol Biomed Life Sci. 2013 Aug 1;932:117-22.
In this study we report a high sensitive method for the simultaneous analysis of LY2334737 (2'-deoxy-2',2'-difluoro-N-(1-oxo-2-propylpentyl)-cytidine), an amide prodrug of gemcitabine (2', 2'-difluoro-deoxycytidine), along with its active drug gemcitabine and its major metabolite dFdU (2',2'-difluoro-deoxyuridine) by LC-MS/MS. Quantification of all three analytes within a single analysis was challenging because the physio-chemical properties of LY2334737 were significantly different from gemcitabine and dFdU and was accomplished by incorporating column-switching. The assay was fully validated to quantify LY2334737 from 0.1 to 100ng/mL, gemcitabine from 0.25 to 100ng/mL and dFdU from 1 to 1000ng/mL in order to cover the diverse concentration ranges expected in clinical samples. A 25-fold dilution was also validated to accommodate any samples outside this range. Overall, the assay had good accuracy (ranging from -7.0 to 1.2% relative error) and precision (ranging from 2.1 to 8.4% relative standard deviation). Extraction efficiency was greater than 80% for all three analytes and there were no matrix effects. Plasma samples were stable for 24h at room temperature, 660 days in frozen storage, and at least 4 freeze-thaw cycles, at both -20 and -70 degrees C. Data from clinical trials showed that plasma concentrations for LY2334737, gemcitabine, and dFdU were successfully quantified from a single LC-MS/MS analysis and that the assay ranges selected for the three analytes were appropriate and minimized the need for reanalysis.
Phase 1b study of the oral gemcitabine 'Pro-drug' LY2334737 in combination with capecitabine in patients with advanced solid tumors.[Pubmed:25640850]
Invest New Drugs. 2015 Apr;33(2):432-9.
Background This Phase 1b study aimed to determine the recommended Phase 2 dose of LY2334737, an oral pro-drug of gemcitabine, in combination with capecitabine, an oral pro-drug of 5-fluorouracil, in patients with advanced solid tumors. In addition, pharmacokinetics (PK) and tumor response were evaluated. Patients and methods Patients with advanced/metastatic solid tumors received 650 mg/m(2) capecitabine twice daily (BID) and escalating doses of LY2334737 once daily (QD; initial dose 10 mg/day), both for 14 days followed by 7-day drug holiday. Cycles were repeated until progressive disease (PD) or unacceptable toxicity. Results Fifteen patients received a median of 2 (range 1-7) treatment cycles; 14 patients discontinued due to PD, 1 due to toxicity (pyrexia). LY2334737 doses up to 40 mg/day were explored. Three dose-limiting toxicities were reported by 2 patients (fatigue, diarrhea, hyponatremia; all Grade 3). Seven patients achieved stable disease. Enrollment was stopped after unexpected hepatic toxicities were observed with LY2334737 QD in a study of Japanese patients. PK parameters for LY2334737 were consistent with the first-in-human study of LY2334737; PK data after 14 day combination treatment revealed no drug-drug interactions between LY2334737 and capecitabine. Conclusions No drug interactions or unexpected toxicities were observed in US patients when LY2334737 at doses up to 40 mg/day was administered QD in combination with capecitabine BID; the maximum tolerated dose was not reached.
Phase I dose escalation and pharmacokinetic evaluation of two different schedules of LY2334737, an oral gemcitabine prodrug, in patients with advanced solid tumors.[Pubmed:26377590]
Invest New Drugs. 2015 Dec;33(6):1206-16.
BACKGROUND: This Phase-I-study aimed to determine the recommended Phase-II-dosing-schedule of LY2334737, an oral gemcitabine prodrug, in patients with advanced/metastatic solid tumors. Pharmacokinetics, cytokeratin-18 (CK18) levels, genetic polymorphisms, and antitumor activity were additionally evaluated. METHODS: Patients received escalating doses of LY2334737 either every other day for 21 days (d) followed by 7 days-drug-free period (QoD-arm) or once daily for 7 days every other week (QD-arm). The 28 days-cycles were repeated until disease progression or unacceptable toxicity. Standard 3 + 3 dose-escalation was succeeded by a dose-confirmation phase (12 additional patients to be enrolled on the maximum tolerated dose [MTD]). RESULTS: Forty-one patients received QoD- (40-100 mg) and 32 QD-dosing (40-90 mg). On QoD, 3/9 patients experienced dose-limiting toxicities (DLTs) on the 100 mg dose (2 x G3 diarrhea, 1 x G3 transaminase increase); 1 additional DLT (G3 diarrhea) occurred during dose confirmation at 90 mg (12 patients). On QD, 1 patient each experienced DLTs on 60 mg (G3 transaminase increase) and 80 mg (G3 prolonged QTcF-interval); 2/7 patients had 3 DLTs on the 90 mg dose (diarrhea, edema, liver-failure; all G3). The MTD was established at 90 mg for the QoD-arm. Seven patients on QoD and 4 on QD achieved SD (no CR + PR). Pharmacokinetics showed a dose-proportional increase in exposure of LY2334737 and dFdC without accumulation after repeated dosing. Significant increases in CK18 levels were observed. Genetic polymorphism of the cytidine deaminase gene (rs818202) could be associated with >/= G3 hepatotoxicity. CONCLUSIONS: Both schedules displayed linear pharmacokinetics and acceptable safety profiles. The recommended dose and schedule of LY2334737 for subsequent Phase-II-studies is 90 mg given QoD for 21 day.


