TolbutamideCAMP inhibitor CAS# 64-77-7 |
- AZD3514
Catalog No.:BCC1070
CAS No.:1240299-33-5
- 17 alpha-propionate
Catalog No.:BCC1296
CAS No.:19608-29-8
- Andarine
Catalog No.:BCC1168
CAS No.:401900-40-1
- MDV3100 (Enzalutamide)
Catalog No.:BCC1268
CAS No.:915087-33-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 64-77-7 | SDF | Download SDF |
| PubChem ID | 5505 | Appearance | Powder |
| Formula | C12H18N2O3S | M.Wt | 270.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 34 mg/mL (125.76 mM) *"≥" means soluble, but saturation unknown. | ||
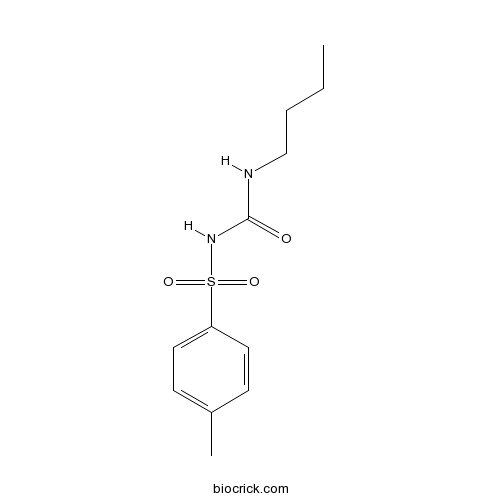
| Chemical Name | 1-butyl-3-(4-methylphenyl)sulfonylurea | ||
| SMILES | CCCCNC(=O)NS(=O)(=O)C1=CC=C(C=C1)C | ||
| Standard InChIKey | JLRGJRBPOGGCBT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H18N2O3S/c1-3-4-9-13-12(15)14-18(16,17)11-7-5-10(2)6-8-11/h5-8H,3-4,9H2,1-2H3,(H2,13,14,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tolbutamide is a first generation potassium channel blocker, sulfonylurea oral hypoglycemic drug.
Target: Potassium Channel
Tolbutamide is an oral antihyperglycemic agent used for the treatment of non-insulin-dependent diabetes mellitus (NIDDM). Tolbutamide act by stimulating β cells of the pancreas to release insulin. Sulfonylureas increase both basal insulin secretion and meal-stimulated insulin release. Tolbutamide belongs to a class of medications called sulfonylureas. Tolbutamide inhibits both the basal and the cyclic AMP-stimulated protein kinase activities and the IC50 of Tolbutamide is 4 mM. Similar Tolbutamide concentrations are required for half maximal inhibition of in vitro lipolysis induced by hormones (norepinephrine and ACTH) or by dibutyryl cyclic AMP plus theophylline. Tolbutamide also inhibits both soluble and membrane-bound protein kinase from canine heart. The Tolbutamide inhibition of adipose tissue cyclic AMP-dependent protein kinase is one possible explanation for the antilipolytic effects of this drug [1]. Tolbutamide inhibits C6-glioma cell proliferation by increasing Cx43, which correlates with a reduction in pRb phosphorylation due to the up-regulation of the Cdk inhibitors p21 and p27 [2]. References: | |||||

Tolbutamide Dilution Calculator

Tolbutamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6989 mL | 18.4945 mL | 36.9891 mL | 73.9782 mL | 92.4727 mL |
| 5 mM | 0.7398 mL | 3.6989 mL | 7.3978 mL | 14.7956 mL | 18.4945 mL |
| 10 mM | 0.3699 mL | 1.8495 mL | 3.6989 mL | 7.3978 mL | 9.2473 mL |
| 50 mM | 0.074 mL | 0.3699 mL | 0.7398 mL | 1.4796 mL | 1.8495 mL |
| 100 mM | 0.037 mL | 0.1849 mL | 0.3699 mL | 0.7398 mL | 0.9247 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tolbutamide is a potent inhibitor of cAMP with an IC50 value of 4mM [1].
Tolbutamide has been reported to inhibit both the basal and the cyclic AMP-stimulated protein kinase activites with an IC50 value of 4mM for cyclic AMP-dependent kinase activity. In addition, Tolbutamide has been revealed to inhibit both soluble and membrane-bound protein kinase from canine heart. Moreover, the Tolbutamide inhibition of adipose tissue cyclic AMP- dependent protein kinase is explanation for antilipolytic effects [1]. Besides, Tolbutamide and dbcAMP has been exhibited to increase about four-fold levels of Cx43 mRNA and decrease about 80% the expression of Ki-67 [2].
References:
[1] Wray HL, Harris AW. Adenosine 3', 5'-monophosphate-dependent protein kinase in adipose tissue: inhibition by tolbutamide. Biochem Biophys Res Commun. 1973 Jul 2;53(1):291-4.
[2] Sánchez-Alvarez R1, Paíno T, Herrero-González S, Medina JM, Tabernero A. Tolbutamide reduces glioma cell proliferation by increasing connexin43, which promotes the up-regulation of p21 and p27 and subsequent changes in retinoblastoma phosphorylation. Glia. 2006 Aug 1;54(2):125-34.
- Tetracycline Hydrochloride
Catalog No.:BCC1206
CAS No.:64-75-5
- Demeclocycline hydrochloride
Catalog No.:BCC5303
CAS No.:64-73-3
- Physostigmine hemisulfate
Catalog No.:BCC6724
CAS No.:64-47-1
- Lobeline Hydrochloride
Catalog No.:BCC8202
CAS No.:63990-84-1
- 13-Oxopodocarp-8(14)-en-18-oic acid
Catalog No.:BCN4009
CAS No.:63976-69-2
- Hopeyhopin
Catalog No.:BCN7533
CAS No.:63975-56-4
- Artemisinin
Catalog No.:BCN5814
CAS No.:63968-64-9
- (3R,10S)-Heptadeca-1,8-diene-4,6-diyne-3,10-diol
Catalog No.:BCC9111
CAS No.:63910-76-9
- VX-680 (MK-0457,Tozasertib)
Catalog No.:BCC2167
CAS No.:639089-54-6
- VUF 5681 dihydrobromide
Catalog No.:BCC7383
CAS No.:639089-06-8
- LH846
Catalog No.:BCC4246
CAS No.:639052-78-1
- Zinc Phytate
Catalog No.:BCN8302
CAS No.:63903-51-5
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- Etimizol
Catalog No.:BCC1562
CAS No.:64-99-3
- H-D-Val-OH
Catalog No.:BCC3145
CAS No.:640-68-6
- Paroxetine maleate
Catalog No.:BCC7265
CAS No.:64006-44-6
- Tamgermanetin
Catalog No.:BCN7944
CAS No.:640235-90-1
- 4(15)-Oppositene-1,7-diol
Catalog No.:BCN4180
CAS No.:640289-58-3
- Torachrysone 8-O-glucoside
Catalog No.:BCN4181
CAS No.:64032-49-1
- Boc-Lys(Ac)-OH
Catalog No.:BCC3411
CAS No.:6404-26-8
- Boc-Nle-OH
Catalog No.:BCC3296
CAS No.:6404-28-0
- Boc-ε-Acp-OH
Catalog No.:BCC3205
CAS No.:6404-29-1
- Z-D-Pro-OH
Catalog No.:BCC2752
CAS No.:6404-31-5
- Ro 04-5595 hydrochloride
Catalog No.:BCC7234
CAS No.:64047-73-0
Thermodynamic Stability Analysis of Tolbutamide Polymorphs and Solubility in Organic Solvents.[Pubmed:27238487]
J Pharm Sci. 2016 Jun;105(6):1901-1906.
Melting temperatures and enthalpies of fusion have been determined by differential scanning calorimetry (DSC) for 2 polymorphs of the drug Tolbutamide: FI(H) and FV. Heat capacities have been determined by temperature-modulated DSC for 4 polymorphs: FI(L), FI(H), FII, FV, and for the supercooled melt. The enthalpy of fusion of FII at its melting point has been estimated from the enthalpy of transition of FII into FI(H) through a thermodynamic cycle. Calorimetric data have been used to derive a quantitative polymorphic stability relationship between these 4 polymorphs, showing that FII is the stable polymorph below approximately 333 K, above which temperature FI(H) is the stable form up to its melting point. The relative stability of FV is well below the other polymorphs. The previously reported kinetic reversibility of the transformation between FI(L) and FI(H) has been verified using in situ Raman spectroscopy. The solid-liquid solubility of FII has been gravimetrically determined in 5 pure organic solvents (methanol, 1-propanol, ethyl acetate, acetonitrile, and toluene) over the temperature range 278 to 323 K. The ideal solubility has been estimated from calorimetric data, and solution activity coefficients at saturation in the 5 solvents determined. All solutions show positive deviation from Raoult's law, and all van't Hoff plots of solubility data are nonlinear. The solubility in toluene is well below that observed in the other investigated solvents. Solubility data have been correlated and extrapolated to the melting point using a semiempirical regression model.
Effect of Temperature on Tolbutamide Binding to Glycated Serum Albumin.[Pubmed:28362348]
Molecules. 2017 Mar 31;22(4). pii: molecules22040569.
Glycation process occurs in protein and becomes more pronounced in diabetes when an increased amount of reducing sugar is present in bloodstream. Glycation of protein may cause conformational changes resulting in the alterations of its binding properties even though they occur at a distance from the binding sites. The changes in protein properties could be related to several pathological consequences such as diabetic and nondiabetic cardiovascular diseases, cataract, renal dysfunction and Alzheimer's disease. The experiment was designed to test the impact of glycation process on sulfonylurea drug Tolbutamide-albumin binding under physiological (T = 309 K) and inflammatory (T = 311 K and T = 313 K) states using fluorescence and UV-VIS spectroscopies. It was found in fluorescence analysis experiments that the modification of serum albumin in tryptophanyl and tyrosyl residues environment may affect the Tolbutamide (TB) binding to albumin in subdomain IIA and/or IIIA (Sudlow's site I and/or II), and also in subdomains IB and IIB. We estimated the binding of Tolbutamide to albumin described by a mixed nature of interaction (specific and nonspecific). The association constants Ka (Lmol(-1)) for Tolbutamide at its high affinity sites on non-glycated albumin were in the range of 1.98-7.88 x 10(4) Lmol(-1) (lambdaex = 275 nm), 1.20-1.64 x 10(4) Lmol(-1) (lambdaex = 295 nm) and decreased to 1.24-0.42 x 10(4) Lmol(-1) at lambdaex = 275 nm (T = 309 K and T = 311 K) and increased to 2.79 x 10(4) Lmol(-1) at lambdaex = 275 nm (T = 313 K) and to 4.43-6.61 x 10(4) Lmol(-1) at lambdaex = 295 nm due to the glycation process. Temperature dependence suggests the important role of van der Waals forces and hydrogen bonding in hydrophobic interactions between Tolbutamide and both glycated and non-glycated albumin. We concluded that the changes in the environment of TB binding of albumin in subdomain IIA and/or IIIA as well as in subdomains IB and IIB influence on therapeutic effect and therefore the studies of the binding of Tolbutamide (in diabetes) to transporting protein under glycation that refers to the modification of a protein are of great importance in pharmacology and biochemistry. This information may lead to the development of more effective drug therapy in people with diabetes.
Evaluation of Pharmacokinetic Interactions Between Lesinurad, a New Selective Urate Reabsorption Inhibitor, and CYP Enzyme Substrates Sildenafil, Amlodipine, Tolbutamide, and Repaglinide.[Pubmed:28067999]
Clin Pharmacol Drug Dev. 2017 Jul;6(4):363-376.
Lesinurad is a selective uric acid reabsorption inhibitor approved for the treatment of hyperuricemia associated with gout in combination with xanthine oxidase inhibitors. In vitro assays indicate that lesinurad is an inducer of CYPs in the order CYP3A > CYP2C8 > CYP2C9 > CYP2C19 > CYP2B6 and an inhibitor of CYP2C8 and CYP2C9. To investigate the drug interaction potential of lesinurad, clinical drug interaction studies were conducted. Open-label studies in volunteers investigated the effects of single-/multiple-dose lesinurad on the pharmacokinetics of sildenafil and amlodipine (CYP3A4 induction), Tolbutamide (CYP2C9 inhibition/induction), and repaglinide (CYP2C8 inhibition/induction). There was no apparent induction of CYP2C8 and CYP2C9 following repeated lesinurad administration, although no inhibition of CYP2C9 and modest inhibition of CYP2C8 were observed following single-dose lesinurad. Consistent with in vitro observations, lesinurad (200 mg once daily) was an inducer of CYP3A based on the effects on sildenafil exposure. Sildenafil exposure decreased by approximately 34% for Cmax and AUC when administered with multiple-dose lesinurad 200 mg and allopurinol 300 mg, relative to sildenafil alone. During lesinurad therapy, the possibility of reduced efficacy of concomitant drugs that are CYP3A substrates should be considered and their efficacy monitored because of induction of CYP3A by lesinurad.
Altered tolbutamide pharmacokinetics by a decrease in hepatic expression of CYP2C6/11 in rats pretreated with 5-fluorouracil.[Pubmed:28051340]
Xenobiotica. 2018 Jan;48(1):53-59.
1. We investigated the change in the pharmacokinetic profile of Tolbutamide (TB), a substrate for CYP2C6/11, 4 days after single administration of 5-fluorouracil (5-FU), and the hepatic gene expression and activity of CYP2C6/11 were also examined in 5-FU-pretreated rats. 2. Regarding the pharmacokinetic parameters of the 5-FU group, the area under the curve (AUC) was significantly increased, and correspondingly, the elimination rate constant at the terminal phase (ke) was significantly decreased without significant change in the volume of distribution at the steady state (Vdss). 3. The metabolic production of 4-hydroxylated TB in hepatic microsomes was significantly reduced by the administration of 5-FU. 4. The expression level of mRNAs for hepatic CYP2C6 and CYP2C11 was significantly lower than in the control group when the rats were pretreated with 5-FU. 5. These results demonstrated that the pharmacokinetic profile of TB was altered by the treatment with 5-FU through a metabolic process, which may be responsible for the decreased CYP2C6/11 expression at mRNA levels.


