MDV3100 (Enzalutamide)Androgen receptor antagonist CAS# 915087-33-1 |
- AZD3514
Catalog No.:BCC1070
CAS No.:1240299-33-5
- RU 58841
Catalog No.:BCC1911
CAS No.:154992-24-2
- Cyproterone Acetate
Catalog No.:BCC3758
CAS No.:427-51-0
- Spironolactone
Catalog No.:BCC4366
CAS No.:52-01-7
- Bicalutamide
Catalog No.:BCC2481
CAS No.:90357-06-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 915087-33-1 | SDF | Download SDF |
| PubChem ID | 15951529 | Appearance | Powder |
| Formula | C21H16F4N4O2S | M.Wt | 464.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | MDV3100 | ||
| Solubility | DMSO : ≥ 50 mg/mL (107.66 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
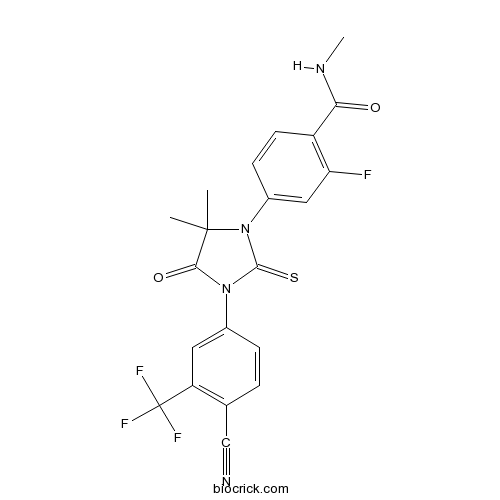
| Chemical Name | 4-[3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-sulfanylideneimidazolidin-1-yl]-2-fluoro-N-methylbenzamide | ||
| SMILES | CC1(C(=O)N(C(=S)N1C2=CC(=C(C=C2)C(=O)NC)F)C3=CC(=C(C=C3)C#N)C(F)(F)F)C | ||
| Standard InChIKey | WXCXUHSOUPDCQV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H16F4N4O2S/c1-20(2)18(31)28(12-5-4-11(10-26)15(8-12)21(23,24)25)19(32)29(20)13-6-7-14(16(22)9-13)17(30)27-3/h4-9H,1-3H3,(H,27,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Enzalutamide (MDV3100) is an antagonist of androgen-receptor (AR) with IC50 of 36 nM. | |||||
| Targets | Androgen-receptor | |||||
| IC50 | 36 nM | |||||
| Cell experiment:[1] | |
| Cell lines | VCaP, LNCaP, 22RV1, DU145 and PC3 prostate cancer cell lines |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 10 μM,12h |
| Applications | Recruitment of AR to target loci was markedly attenuated by MDV3100 and less so by bicalutamide. Interestingly, JQ1 blocked AR recruitment almost as effectively as MDV3100. Limiting our evaluationto AR and BRD4 coincident peaks, we observed that DHT-mediated AR recruitment to these loci was inhibited by MDV3100 and to a lesser extent by JQ1. Corroborating the ChIP seq data, gene expression analysis in VCaP and LNCaP cells showed more efficient repression of DHT-induced AR-target genes by JQ1 than by MDV3100 or bicalutamide. |
| Animal experiment:[1] | |
| Animal models | Four-week-old male SCIDC.B17 mice |
| Dosage form | 10 mg/kg,oral gavage or intraperitonially,five days a week |
| Application | Treatment of VCaP tumour-bearing mice with JQ1 led to a significant reduction in tumour volume/weight, whereas MDV3100 had a less pronounced effect. Recently, several studies described the pro-metastatic effects of MDV3100 in pre-clinical models. To test whether MDV3100 treatment leads to spontaneous metastasis in our VCaP xenograft model, we isolated femur, liver and spleen from MDV3100-treated mice and found evidence of metastases in femur and liver. By contrast, JQ1-treated mice showed no evidence of metastasis. Taken together, these pre-clinical studies suggest that the use of MDV3100 in clinically localized prostate cancer may potentiate the formation of micro-metastases, unlike BET inhibitors. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| Phase III experiment [2]: | |
| Target | Men with castration-resistant prostate cancer |
| Dosage form | 160 mg per day (800 patients) or placebo (399 patients) |
| Application | The superiority of enzalutamide over placebo was shown with respect to all secondary end points: the proportion of patients with a reduction in the prostate-specific antigen (PSA) level by 50% or more (54% vs. 2%, P<0.001), the soft-tissue response rate (29% vs. 4%, P<0.001), the quality-of-life response rate (43% vs. 18%, P<0.001), the time to PSA progression (8.3 vs. 3.0 months; hazard ratio, 0.25; P<0.001), radiographic progression-free survival (8.3 vs. 2.9 months; hazard ratio, 0.40; P<0.001), and the time to the first skeletal-related event (16.7 vs. 13.3 months; hazard ratio, 0.69; P<0.001). Rates of fatigue, diarrhea, and hot flashes were higher in the enzalutamide group. |
| References: 1. Asangani IA, Dommeti VL, Wang X et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014 Jun 12;510(7504):278-82. 2. Scher HI, Fizazi K, Saad F et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012 Sep 27;367(13):1187-97. | |

MDV3100 (Enzalutamide) Dilution Calculator

MDV3100 (Enzalutamide) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1533 mL | 10.7666 mL | 21.5332 mL | 43.0663 mL | 53.8329 mL |
| 5 mM | 0.4307 mL | 2.1533 mL | 4.3066 mL | 8.6133 mL | 10.7666 mL |
| 10 mM | 0.2153 mL | 1.0767 mL | 2.1533 mL | 4.3066 mL | 5.3833 mL |
| 50 mM | 0.0431 mL | 0.2153 mL | 0.4307 mL | 0.8613 mL | 1.0767 mL |
| 100 mM | 0.0215 mL | 0.1077 mL | 0.2153 mL | 0.4307 mL | 0.5383 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
As an approved agent for the treatment of mCRPC, enzalutamide, an oral inhibitor of androgen receptor, does not require administration with steroids and was well tolerated in randomized phase III AFFIRM study.
Abstract
The cross-resistance between taxanes (docetaxel and cabazitaxel) and new hormonal agents (abiraterone and enzalutamide) and their effects on AR nuclear translocation have been investigated.
Abstract
Enzlutamide is an inhibitor of AR signaling that inhibits multiple steps of AR signaling, blocks the growth of CRPC cells and increases survival in patients with metastatic CRPC.
Abstract
In preclinical studies, enzalutamide blocks androgen signaling and the bind of AR to DNA leading to apoptosis and slowed tumor growth; while, in clinical trials, it exhibited significant antitumor activity with an optimal safety profile and significantly improved survival in metastatic CRPC male patients with prior chemotherapy.
Abstract
Although Abiraterone acetate and enzalutamide improve outcomes in metastatic mCRPC patients individually, optimal sequencing and occurrence of cross-resistance in combination therapy are unknown.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MDV3100, known as Enzalutamide, is a second-generation androgen receptor (AR) signaling inhibitor. It has been demonstrated impressive affinity to the AR compared to the first-generation AR inhibitors. It is able to inhibit binding of androgens to the AR, AR nuclear translocation, and the association of the AR with DNA. The AR is a 919-amino acid member of the steroid receptor transcription factor superfamily with different domains including an N-terminal regulation domain, a central DNA binding domain, and a C-terminal domain, which includes the ligand-binding domain incorporated within its protein structure. MDV3100 was identified by the Sawyers/Jung laboratories by using the nonsteroidal agonist. Testing was showing that it induced apoptosis in VCaP cells, an AR gene amplified human prostate cancer line, while bicalutamide was ineffective.
Reference
Howard I. Scher, Karim Fizazi, Fred Saad, Mary-Ellen Taplin, Cora N. Sternberg, Kurt Miller, Ronald De Wit, Peter Mulders, Mohammad Hirmand, Bryan Selby, Johann Sebastian. Effect of MDV3100, an androgen receptor signaling inhibitor (ARSI), on overall survival in patients with prostate cancer postdocetaxel: Results from the phase III AFFIRM study. Journal of Clinical Oncology. 2012; 30(5):
Manoj P. Menon, Celestia S. Higano. Enzalutamide, a Second Generation Androgen Receptor Antagonist: Development and Clinical Applications in Prostate Cancer. Current Oncology Reports. 2013; 15(2): 69 – 75.
Joelle El-Amm, Nihar Patel, Ashley Freeman, Jeanny B. Aragon-Ching. Metastatic Castration-Resistant Prostate Cancer: Critical Review of Enzalutamide. Clinical Medicine Insights: Oncology. 2013; 7: 235 – 245.
- BEZ235 (NVP-BEZ235)
Catalog No.:BCC3655
CAS No.:915019-65-7
- Palomid 529
Catalog No.:BCC3905
CAS No.:914913-88-5
- INCB024360 analogue
Catalog No.:BCC1647
CAS No.:914471-09-3
- R788 disodium hexahydrate
Catalog No.:BCC5127
CAS No.:914295-16-2
- 9-Aminocamptothecin
Catalog No.:BCN2453
CAS No.:91421-43-1
- 9-Nitrocamptothecin
Catalog No.:BCN8448
CAS No.:91421-42-0
- 25-O-ethylcimigenol-3-O-beta-D-xylopyranoside
Catalog No.:BCN1309
CAS No.:914086-57-0
- PluriSIn #1 (NSC 14613)
Catalog No.:BCC2305
CAS No.:91396-88-2
- Lu AA 47070
Catalog No.:BCC7977
CAS No.:913842-25-8
- CS 2100
Catalog No.:BCC6221
CAS No.:913827-99-3
- SC75741
Catalog No.:BCC5448
CAS No.:913822-46-5
- Ropinirole HCl
Catalog No.:BCC4939
CAS No.:91374-20-8
- 3-[4-[(2-Chloro-5-iodophenyl)methyl]phenoxy]tetrahydro-furan
Catalog No.:BCC8601
CAS No.:915095-94-2
- Bakkenolide IIIa
Catalog No.:BCN7352
CAS No.:915289-60-0
- Cot inhibitor-2
Catalog No.:BCC1497
CAS No.:915363-56-3
- Cot inhibitor-1
Catalog No.:BCC1496
CAS No.:915365-57-0
- ABC294640
Catalog No.:BCC4192
CAS No.:915385-81-8
- WAY 316606
Catalog No.:BCC2052
CAS No.:915759-45-4
- Dovitinib (TKI258) Lactate
Catalog No.:BCC6473
CAS No.:915769-50-5
- Momordicine I
Catalog No.:BCN3058
CAS No.:91590-76-0
- Benidipine HCl
Catalog No.:BCC4395
CAS No.:91599-74-5
- TCS 1102
Catalog No.:BCC4063
CAS No.:916141-36-1
- AC 55541
Catalog No.:BCC3951
CAS No.:916170-19-9
- Gopherenediol
Catalog No.:BCN6582
CAS No.:916236-79-8
Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment.[Pubmed:24382803]
Cancer. 2014 Apr 1;120(7):968-75.
BACKGROUND: Enzalutamide (Enz) and abiraterone acetate (AA) are hormone treatments that have a proven survival advantage in patients with metastatic, castration-resistant prostate cancer who previously received docetaxel (Doc). Recently, limited activity of AA after Enz and of Enz after AA was demonstrated in small cohort studies. Here, the authors present the activity and tolerability of Enz in patients who previously received AA and Doc in the largest cohort to date. METHODS: The efficacy and tolerability of Enz were investigated in men with progressive, metastatic, castrate-resistant prostate cancer who previously received Doc and AA. Toxicity, progression-free survival, time to prostate-specific antigen (PSA) progression, and overall survival were retrospectively evaluated. RESULTS: Sixty-one patients were included in the analysis. The median age was 69 years (interquartile range [IQR], 64-74 years), 57 patients (93%) had an Eastern Cooperative Oncology Group performance status from 0 to 2, 48 patients (79%) had bone metastases, 33 patients (54%) had lymph node metastases, and 13 patients (21%) had visceral metastases. The median duration of Enz treatment was 14.9 weeks (IQR, 11.1-20.0 weeks), and 13 patients (21%) had a maximum PSA decline >/=50%. The median progression-free survival was 12.0 weeks (95% confidence interval [CI], 11.1-16.0 weeks), the median time to PSA progression was 17.4 weeks (95% CI, >16.0 weeks), and the median overall survival was 31.6 weeks (95% CI, >28.7 weeks). Enz was well tolerated, and fatigue and musculoskeletal pain were the most frequent grade >/=2 adverse events. The PSA response to Doc and AA did not predict the PSA response to Enz. CONCLUSIONS: Enz has modest clinical activity in patients with metastatic, castrate-resistant prostate cancer who previously received Doc and AA. PSA response to Doc and AA does not predict for PSA response to ENz.
Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone.[Pubmed:24074764]
Eur J Cancer. 2014 Jan;50(1):78-84.
BACKGROUND: The new generation anti-androgen enzalutamide and the potent CYP17 inhibitor abiraterone have both demonstrated survival benefits in patients with metastatic castration-resistant prostate cancer (CRPC) progressing after docetaxel. Preliminary data on the antitumour activity of abiraterone after enzalutamide have suggested limited activity. The antitumour activity and safety of enzalutamide after abiraterone in metastatic CRPC patients is still unknown. PATIENTS AND METHODS: We retrospectively identified patients treated with docetaxel and abiraterone prior to enzalutamide to investigate the activity and safety of enzalutamide in a more advanced setting. Prostate specific antigen (PSA), radiological and clinical assessments were analysed. RESULTS: 39 patients with metastatic CRPC were identified for this analysis (median age 70years, range: 54-85years). Overall 16 patients (41%) had a confirmed PSA decline of at least 30%. Confirmed PSA declines of 50% and 90% were achieved in 5/39 (12.8%) and 1/39 (2.5%) respectively. Of the 15 patients who responded to abiraterone, two (13.3%) also had a confirmed 50% PSA decline on subsequent enzalutamide. Among the 22 abiraterone-refractory patients, two (9%) achieved a confirmed 50% PSA decline on enzalutamide. CONCLUSION: Our preliminary case series data suggest limited activity of enzalutamide in the post-docetaxel and post-abiraterone patient population.
An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide).[Pubmed:23842682]
Cancer Discov. 2013 Sep;3(9):1030-43.
UNLABELLED: Castration-resistant prostate cancer (CRPC) is the most aggressive, incurable form of prostate cancer. MDV3100 (Enzalutamide), an antagonist of the androgen receptor (AR), was approved for clinical use in men with metastatic CRPC. Although this compound showed clinical efficacy, many initial responders later developed resistance. To uncover relevant resistant mechanisms, we developed a model of spontaneous resistance to MDV3100 in LNCaP prostate cancer cells. Detailed characterization revealed that emergence of an F876L mutation in AR correlated with blunted AR response to MDV3100 and sustained proliferation during treatment. Functional studies confirmed that AR(F876L) confers an antagonist-to-agonist switch that drives phenotypic resistance. Finally, treatment with distinct antiandrogens or cyclin-dependent kinase (CDK)4/6 inhibitors effectively antagonized AR(F876L) function. Together, these findings suggest that emergence of F876L may (i) serve as a novel biomarker for prediction of drug sensitivity, (ii) predict a "withdrawal" response to MDV3100, and (iii) be suitably targeted with other antiandrogens or CDK4/6 inhibitors. SIGNIFICANCE: We uncovered an F876L agonist-switch mutation in AR that confers genetic and phenotypic resistance to the antiandrogen drug MDV3100. On the basis of this fi nding, we propose new therapeutic strategies to treat patients with prostate cancer presenting with this AR mutation.
Anti-androgen receptor ASC-J9 versus anti-androgens MDV3100 (Enzalutamide) or Casodex (Bicalutamide) leads to opposite effects on prostate cancer metastasis via differential modulation of macrophage infiltration and STAT3-CCL2 signaling.[Pubmed:23928703]
Cell Death Dis. 2013 Aug 8;4:e764.
Despite androgen deprivation therapy (ADT) suppression of prostate cancer (PCa) growth, its overall effects on PCa metastasis remain unclear. Using human (C4-2B/THP1) and mouse (TRAMP-C1/RAW264.7) PCa cells-macrophages co-culture systems, we found currently used anti-androgens, MDV3100 (Enzalutamide) or Casodex (bicalutamide), promoted macrophage migration to PCa cells that consequently led to enhanced PCa cell invasion. In contrast, the AR degradation enhancer, ASC-J9, suppressed both macrophage migration and subsequent PCa cell invasion. Mechanism dissection showed that Casodex/MDV3100 reduced the AR-mediated PIAS3 expression and enhanced the pSTAT3-CCL2 pathway. Addition of CCR2 antagonist reversed the Casodex/MDV3100-induced macrophage migration and PCa cell invasion. In contrast, ASC-J9 could regulate pSTAT3-CCL2 signaling using two pathways: an AR-dependent pathway via inhibiting PIAS3 expression and an AR-independent pathway via direct inhibition of the STAT3 phosphorylation/activation. These findings were confirmed in the in vivo mouse model with orthotopically injected TRAMP-C1 cells. Together, these results may raise the potential concern about the currently used ADT with anti-androgens that promotes PCa metastasis and may provide some new and better therapeutic strategies using ASC-J9 alone or a combinational therapy that simultaneously targets androgens/AR signaling and PIAS3-pSTAT3-CCL2 signaling to better battle PCa growth and metastasis at castration-resistant stage.


