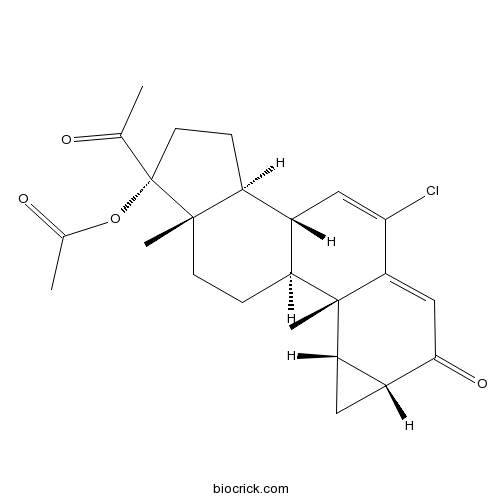
Cyproterone AcetateAndrogen receptor antagonist CAS# 427-51-0 |
- AZD3514
Catalog No.:BCC1070
CAS No.:1240299-33-5
- 17 alpha-propionate
Catalog No.:BCC1296
CAS No.:19608-29-8
- Andarine
Catalog No.:BCC1168
CAS No.:401900-40-1
- MDV3100 (Enzalutamide)
Catalog No.:BCC1268
CAS No.:915087-33-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 427-51-0 | SDF | Download SDF |
| PubChem ID | 9880 | Appearance | Powder |
| Formula | C24H29ClO4 | M.Wt | 416.94 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 33.33 mg/mL (82.72 mM; Need ultrasonic) Ethanol : 20 mg/mL (49.64 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| SMILES | CC(=O)C1(CCC2C1(CCC3C2C=C(C4=CC(=O)C5CC5C34C)Cl)C)OC(=O)C | ||
| Standard InChIKey | UWFYSQMTEOIJJG-FDTZYFLXSA-N | ||
| Standard InChI | InChI=1S/C24H29ClO4/c1-12(26)24(29-13(2)27)8-6-16-14-10-20(25)19-11-21(28)15-9-18(15)23(19,4)17(14)5-7-22(16,24)3/h10-11,14-18H,5-9H2,1-4H3/t14-,15+,16-,17-,18-,22-,23-,24-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cyproterone Acetate Dilution Calculator

Cyproterone Acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3984 mL | 11.9921 mL | 23.9843 mL | 47.9685 mL | 59.9607 mL |
| 5 mM | 0.4797 mL | 2.3984 mL | 4.7969 mL | 9.5937 mL | 11.9921 mL |
| 10 mM | 0.2398 mL | 1.1992 mL | 2.3984 mL | 4.7969 mL | 5.9961 mL |
| 50 mM | 0.048 mL | 0.2398 mL | 0.4797 mL | 0.9594 mL | 1.1992 mL |
| 100 mM | 0.024 mL | 0.1199 mL | 0.2398 mL | 0.4797 mL | 0.5996 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cyproterone acetate has antagonistic properties towards the androgen receptor with an IC50 value of 7.1 nM. It can however, also act as a partial agonist of AR at an EC50 of 4.0 μM.
- S-Isopropylisothiourea hydrobromide
Catalog No.:BCC6837
CAS No.:4269-97-0
- NS 5806
Catalog No.:BCC7872
CAS No.:426834-69-7
- 4-Hydroxy-3-methoxyphenyl O-beta-D-6-O-syringate-glucopyranoside
Catalog No.:BCN1442
CAS No.:426821-85-4
- Hemapolin
Catalog No.:BCC8994
CAS No.:4267-80-5
- Dehydrodiconiferyl alcohol
Catalog No.:BCN6878
CAS No.:4263-87-0
- 21,23-Dihydro-23-hydroxy-21-oxozapoterin
Catalog No.:BCN7230
CAS No.:426266-88-8
- TAK-700 salt
Catalog No.:BCC1979
CAS No.:426219-53-6
- TAK-700 (Orteronel)
Catalog No.:BCC2280
CAS No.:426219-18-3
- Luteolin-6-C-glucoside
Catalog No.:BCN4985
CAS No.:4261-42-1
- 8-Hydroxy-5-O-beta-D-glucopyranosylpsoralen
Catalog No.:BCN1443
CAS No.:425680-98-4
- Sotrastaurin (AEB071)
Catalog No.:BCC3857
CAS No.:425637-18-9
- Crocin
Catalog No.:BCN2373
CAS No.:42553-65-1
- Pedunculoside
Catalog No.:BCN1191
CAS No.:42719-32-4
- 2-Aminophenyl phenyl sulfone
Catalog No.:BCC8554
CAS No.:4273-98-7
- D-Valinol
Catalog No.:BCC2693
CAS No.:4276-09-9
- Magnoflorine Iodide
Catalog No.:BCN2911
CAS No.:4277-43-4
- H-N-Me-Leu-OBzl.TosOH
Catalog No.:BCC3215
CAS No.:42807-66-9
- Shinjulactone L
Catalog No.:BCN7958
CAS No.:4283-49-2
- Catechin 7-xyloside
Catalog No.:BCN5484
CAS No.:42830-48-8
- Flumequine
Catalog No.:BCC5090
CAS No.:42835-25-6
- Flumequine sodium
Catalog No.:BCC8985
CAS No.:42835-68-7
- H-Ala-OBzl.TosOH
Catalog No.:BCC3191
CAS No.:42854-62-6
- 2-(3-Benzoylphenyl)propionitrile
Catalog No.:BCC8479
CAS No.:42872-30-0
- For-Met-OH
Catalog No.:BCC2992
CAS No.:4289-98-9
Cyproterone acetate enhances TRAIL-induced androgen-independent prostate cancer cell apoptosis via up-regulation of death receptor 5.[Pubmed:28270124]
BMC Cancer. 2017 Mar 7;17(1):179.
BACKGROUND: Virtually all prostate cancer deaths occur due to obtaining the castration-resistant phenotype after prostate cancer cells escaped from apoptosis and/or growth suppression initially induced by androgen receptor blockade. TNF-related apoptosis-inducing ligand (TRAIL) was an attractive cancer therapeutic agent due to its minimal toxicity to normal cells and remarkable apoptotic activity in tumor cells. However, most localized cancers including prostate cancer are resistant to TRAIL-induced apoptosis, thereby creating a therapeutic challenge of inducing TRAIL sensitivity in cancer cells. Herein the effects of Cyproterone Acetate, an antiandrogen steroid, on the TRAIL-induced apoptosis of androgen receptor-negative prostate cancer cells are reported. METHODS: Cell apoptosis was assessed by both annexin V/propidium iodide labeling and poly (ADP-ribose) polymerase cleavage assays. Gene and protein expression changes were determined by quantitative real-time PCR and western blot assays. The effect of Cyproterone Acetate on gene promoter activity was determined by luciferase reporter assay. RESULTS: Cyproterone Acetate but not AR antagonist bicalutamide dramatically increased the susceptibility of androgen receptor-negative human prostate cancer PC-3 and DU145 cells to TRAIL-induced apoptosis but no effects on immortalized human prostate stromal PS30 cells and human embryonic kidney HEK293 cells. Further investigation of the TRAIL-induced apoptosis pathway revealed that Cyproterone Acetate exerted its effect by selectively increasing death receptor 5 (DR5) mRNA and protein expression. Cyproterone Acetate treatment also increased DR5 gene promoter activity, which could be abolished by mutation of a consensus binding domain of transcription factor CCAAT-enhancer-binding protein homologous protein (CHOP) in the DR5 gene promoter. Cyproterone Acetate increases CHOP expression in a concentration and time-dependent manner and endoplasmic reticulum stress reducer 4-phenylbutyrate could block Cyproterone Acetate-induced CHOP and DR5 up-regulation. More importantly, siRNA silencing of CHOP significantly reduced Cyproterone Acetate-induced DR5 up-regulation and TRAIL sensitivity in prostate cancer cells. CONCLUSIONS: Our study shows a novel effect of Cyproterone Acetate on apoptosis pathways in prostate cancer cells and raises the possibility that a combination of TRAIL with Cyproterone Acetate could be a promising strategy for treating castration-resistant prostate cancer.
Effects of antiandrogenic progestins, chlormadinone and cyproterone acetate, and the estrogen 17alpha-ethinylestradiol (EE2), and their mixtures: Transactivation with human and rainbowfish hormone receptors and transcriptional effects in zebrafish (Danio rerio) eleuthero-embryos.[Pubmed:27907851]
Aquat Toxicol. 2017 Jan;182:142-162.
Synthetic progestins act as endocrine disrupters in fish but their risk to the environment is not sufficiently known. Here, we focused on an unexplored antiandrogenic progestin, chlormadinone acetate (CMA), and the antiandrogenic progestin Cyproterone Acetate (CPA). The aim was to evaluate whether their in vitro interaction with human and rainbowfish (Melanotaenia fluviatilis) sex hormone receptors is similar. Furthermore, we investigated their activity in zebrafish (Danio rerio) eleuthero-embryos. First, we studied agonistic and antagonistic activities of CMA, CPA, and 17alpha-ethinylestradiol (EE2), in recombinant yeast expressing either the human progesterone (PGR), androgen (AR), or estrogen receptor. The same compounds were also investigated in vitro in a stable transfection cell system expressing rainbowfish nuclear steroid receptors. For human receptors, both progestins exhibited progestogenic, androgenic and antiestrogenic activity with no antiandrogenic or estrogenic activity. In contrast, interactions with rainbowfish receptors showed no progestogenic, but antiandrogenic, antiglucocorticoid, and some antiestrogenic activity. Thus, interaction with and transactivation of human and rainbowfish PGR and AR were distinctly different. Second, we analyzed transcriptional alterations in zebrafish eleuthero-embryos at 96 and 144h post fertilization after exposure to CPA, CMA, EE2, and binary mixtures of CMA and CPA with EE2, mimicking the use in oral contraceptives. CMA led to slight down-regulation of the ar transcript, while CPA down-regulated ar and pgr transcripts. EE2 exposure resulted in significant transcriptional alterations of several genes, including esr1, pgr, vtg1, cyp19b, and gonadotropins (fshb, lhb). The mixture activity of CMA and EE2 followed the independent action model, while CPA and EE2 mixtures showed additive action in transcriptional alterations. Third, we analyzed the interactions of binary mixtures of CMA and CPA, and of CMA and EE2 for their joint activity in vitro and in eleuthero-embryos. Both mixtures behaved according to the concentration addition model in their in vitro interaction with human and rainbowfish receptors, often showing antagonism. In zebrafish eleuthero-embryos, binary mixtures of CMA and EE2 showed the same expression patterns as EE2 alone, indicating an independent action in vivo. Our study demonstrates that CMA and CPA interact distinctly with human and rainbowfish receptors, suggesting that activities of these and possibly additional environmental steroids determined with yeast expressing human receptors cannot simply be translated to fish. The lack of agonistic activities of both progestins to rainbowfish PGR and AR is the probable reason for the low activity found in zebrafish eleuthero-embryos.


