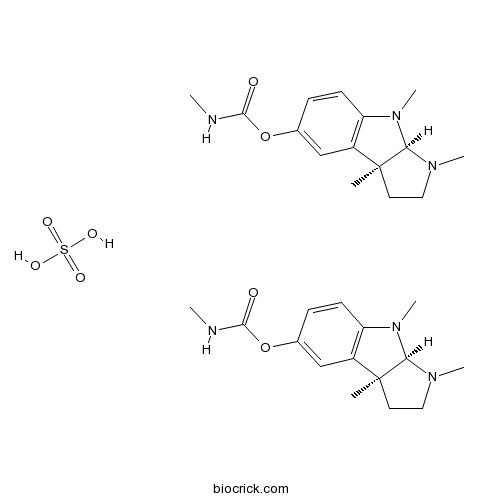
Physostigmine hemisulfateCholinesterase inhibitor CAS# 64-47-1 |
- GDC-0068 (RG7440)
Catalog No.:BCC1271
CAS No.:1001264-89-6
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
- AZD5363
Catalog No.:BCC1073
CAS No.:1143532-39-1
- A-443654
Catalog No.:BCC1321
CAS No.:552325-16-3
- AKT inhibitor VIII
Catalog No.:BCC1334
CAS No.:612847-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 64-47-1 | SDF | Download SDF |
| PubChem ID | 6419928 | Appearance | Powder |
| Formula | C30H44N6O8S | M.Wt | 648.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | (3aS)-cis-1,2,3,3a,8,8a-Hexahydro-1 | ||
| SMILES | CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1.CNC(=O)Oc4ccc5N(C)[C@H]6N(C)CC[C@@]6(C)c5c4.O[S](O)(=O)=O | ||
| Standard InChIKey | YYBNDIVPHIWTPK-KYJQVDHRSA-N | ||
| Standard InChI | InChI=1S/2C15H21N3O2.H2O4S/c2*1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2;1-5(2,3)4/h2*5-6,9,13H,7-8H2,1-4H3,(H,16,19);(H2,1,2,3,4)/t2*13-,15+;/m11./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cholinesterase inhibitor. |

Physostigmine hemisulfate Dilution Calculator

Physostigmine hemisulfate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5413 mL | 7.7065 mL | 15.4131 mL | 30.8261 mL | 38.5327 mL |
| 5 mM | 0.3083 mL | 1.5413 mL | 3.0826 mL | 6.1652 mL | 7.7065 mL |
| 10 mM | 0.1541 mL | 0.7707 mL | 1.5413 mL | 3.0826 mL | 3.8533 mL |
| 50 mM | 0.0308 mL | 0.1541 mL | 0.3083 mL | 0.6165 mL | 0.7707 mL |
| 100 mM | 0.0154 mL | 0.0771 mL | 0.1541 mL | 0.3083 mL | 0.3853 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lobeline Hydrochloride
Catalog No.:BCC8202
CAS No.:63990-84-1
- 13-Oxopodocarp-8(14)-en-18-oic acid
Catalog No.:BCN4009
CAS No.:63976-69-2
- Hopeyhopin
Catalog No.:BCN7533
CAS No.:63975-56-4
- Artemisinin
Catalog No.:BCN5814
CAS No.:63968-64-9
- (3R,10S)-Heptadeca-1,8-diene-4,6-diyne-3,10-diol
Catalog No.:BCC9111
CAS No.:63910-76-9
- VX-680 (MK-0457,Tozasertib)
Catalog No.:BCC2167
CAS No.:639089-54-6
- VUF 5681 dihydrobromide
Catalog No.:BCC7383
CAS No.:639089-06-8
- LH846
Catalog No.:BCC4246
CAS No.:639052-78-1
- Zinc Phytate
Catalog No.:BCN8302
CAS No.:63903-51-5
- Pinoresinol diglucoside
Catalog No.:BCN1093
CAS No.:63902-38-5
- Akuammidine
Catalog No.:BCN6509
CAS No.:639-36-1
- Ajmalidine
Catalog No.:BCN3491
CAS No.:639-30-5
- Demeclocycline hydrochloride
Catalog No.:BCC5303
CAS No.:64-73-3
- Tetracycline Hydrochloride
Catalog No.:BCC1206
CAS No.:64-75-5
- Tolbutamide
Catalog No.:BCC5001
CAS No.:64-77-7
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- Etimizol
Catalog No.:BCC1562
CAS No.:64-99-3
- H-D-Val-OH
Catalog No.:BCC3145
CAS No.:640-68-6
- Paroxetine maleate
Catalog No.:BCC7265
CAS No.:64006-44-6
- Tamgermanetin
Catalog No.:BCN7944
CAS No.:640235-90-1
- 4(15)-Oppositene-1,7-diol
Catalog No.:BCN4180
CAS No.:640289-58-3
- Torachrysone 8-O-glucoside
Catalog No.:BCN4181
CAS No.:64032-49-1
- Boc-Lys(Ac)-OH
Catalog No.:BCC3411
CAS No.:6404-26-8
- Boc-Nle-OH
Catalog No.:BCC3296
CAS No.:6404-28-0
Acetylcholinesterase inhibition and locomotor function after motor-sensory cortex impact injury.[Pubmed:21787180]
J Neurotrauma. 2011 Sep;28(9):1909-19.
Traumatic brain injury (TBI) induces transient or persistent dysfunction of gait and balance. Enhancement of cholinergic transmission has been reported to accelerate recovery of cognitive function after TBI, but the effects of this intervention on locomotor activity remain largely unexplored. The hypothesis that enhancement of cholinergic function by inhibition of acetylcholinesterase (AChE) improves locomotion following TBI was tested in Sprague-Dawley male rats after a unilateral controlled cortical impact (CCI) injury of the motor-sensory cortex. Locomotion was tested by time to fall on the constant speed and accelerating Rotarod, placement errors and time to cross while walking through a horizontal ladder, activity monitoring in the home cages, and rearing behavior. Assessments were performed the 1st and 2nd day and the 1st, 2nd, and 3rd week after TBI. The AChE inhibitor Physostigmine hemisulfate (PHY) was administered continuously via osmotic minipumps implanted subcutaneously at the rates of 1.6-12.8 mumol/kg/day. All measures of locomotion were impaired by TBI and recovered to initial levels between 1 and 3 weeks post-TBI, with the exception of the maximum speed achievable on the accelerating Rotarod, as well as rearing in the open field. PHY improved performance in the accelerating Rotarod at 1.6 and 3.2 mumol/kg/day (AChE activity 95 and 78% of control, respectively), however, higher doses induced progressive deterioration. No effect or worsening of outcomes was observed at all PHY doses for home cage activity, rearing, and horizontal ladder walking. Potential benefits of cholinesterase inhibition on locomotor function have to be weighed against the evidence of the narrow range of useful doses.
Micronized emulsion for controlled release of physostigmine after oral administration. Part I. Formulation design.[Pubmed:2765106]
Drug Des Deliv. 1989 Mar;4(2):135-42.
Our aim was to incorporate physostigmine in a fine micronized emulsion delivery system which would prolong drug release following oral administration. Investigation of various types of equipment and experimental conditions led to a fine micronized emulsion of mean droplet size around 1 micron, which was stable at pH 5.5. The effect of physostigmine concentration and salt formation on the interfacial tension and zeta potential of the emulsion was studied. Physostigmine base markedly decreased the interfacial tension as compared to Physostigmine hemisulfate and salicylate. Zeta potential was highest in the case of the salicylate. After storage at 4 37 degrees C for four months, the emulsion did not undergo any significant change.
Anticholinesterases: medical applications of neurochemical principles.[Pubmed:7722478]
J Neurochem. 1995 May;64(5):1909-18.
Cholinesterases form a family of serine esterases that arise in animals from at least two distinct genes. Multiple forms of these enzymes can be precisely localized and regulated by alternative mRNA splicing and by co- or posttranslational modifications. The high catalytic efficiency of the cholinesterases is quelled by certain very selective reversible and irreversible inhibitors. Owing largely to the important role of acetylcholine hydrolysis in neurotransmission, cholinesterase and its inhibitors have been studied extensively in vivo. In parallel, there has emerged an equally impressive enzyme chemistry literature. Cholinesterase inhibitors are used widely as pesticides; in this regard the compounds are beneficial with concomitant health risks. Poisoning by such compounds can result in an acute but usually manageable medical crisis and may damage the CNS and the PNS, as well as cardiac and skeletal muscle tissue. Some inhibitors have been useful for the treatment of glaucoma and myasthenia gravis, and others are in clinical trials as therapy for Alzheimer's dementia. Concurrently, the most potent inhibitors have been developed as highly toxic chemical warfare agents. We review treatments and sequelae of exposure to selected anticholinesterases, especially organophosphorus compounds and carbamates, as they relate to recent progress in enzyme chemistry.


