Pinoresinol diglucosideCAS# 63902-38-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 63902-38-5 | SDF | Download SDF |
| PubChem ID | 174003 | Appearance | Powder |
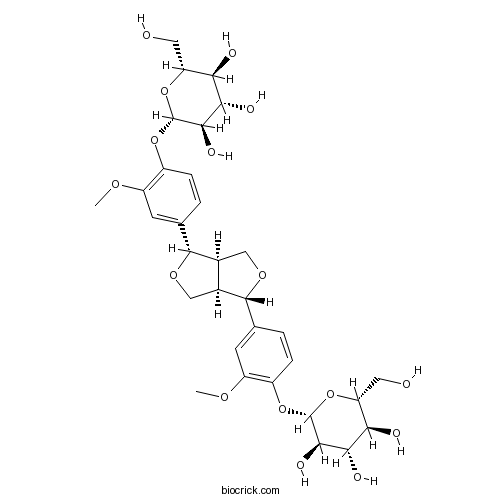
| Formula | C32H42O16 | M.Wt | 682.67 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 6.9 mg/mL (10.11 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2S,3R,4S,5S,6R)-2-[4-[(3S,3aR,6S,6aR)-6-[3-methoxy-4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-1,3,3a,4,6,6a-hexahydrofuro[3,4-c]furan-3-yl]-2-methoxyphenoxy]-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | COC1=C(C=CC(=C1)C2C3COC(C3CO2)C4=CC(=C(C=C4)OC5C(C(C(C(O5)CO)O)O)O)OC)OC6C(C(C(C(O6)CO)O)O)O | ||
| Standard InChIKey | ZJSJQWDXAYNLNS-FUPWJLLWSA-N | ||
| Standard InChI | InChI=1S/C32H42O16/c1-41-19-7-13(3-5-17(19)45-31-27(39)25(37)23(35)21(9-33)47-31)29-15-11-44-30(16(15)12-43-29)14-4-6-18(20(8-14)42-2)46-32-28(40)26(38)24(36)22(10-34)48-32/h3-8,15-16,21-40H,9-12H2,1-2H3/t15-,16-,21+,22+,23+,24+,25-,26-,27+,28+,29+,30+,31+,32+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pinoresinol diglucoside is a putative α-glucosidase inhibiting compound, has antihypertensive activity. It is a heat shock factor 1 inducer, increased expression of HSF1 by a factor of 1.041 at 3 μM. |
| Targets | HSF-1 | α-glucosidase |
| In vitro | Heat shock factor 1 inducers from the bark of Eucommia ulmoides as cytoprotective agents.[Pubmed: 23847077]Chem Biodivers. 2013 Jul;10(7):1322-7.The barks of Eucommia ulmoides (Eucommiae Cortex, Eucommiaceae) have been used as a traditional medicine in Korea, Japan, and China to treat hypertension, reinforce the muscles and bones, and recover the damaged liver and kidney functions.
|
| Kinase Assay | Pinoresinol Diglucoside is Screened as a Putative alpha-Glucosidase Inhibiting Compound in Actinidia arguta leaves[Reference: WebLink]J. Korean Soc. Appl. Bi., 2014, 57(4):473-9.Actinidia arguta leaves are consumed as a popular food material in Korea and have been reported to exert beneficial effects on humans due to its constituent polyphenolic compounds.
In this study, the alpha-glucosidase inhibitory compounds in A. arguta were screened and identified through alpha-glucosidase-guided fractionation and metabolomic analysis.
|
| Structure Identification | Appl Microbiol Biotechnol. 2012 Feb;93(4):1475-83.Structure identification and fermentation characteristics of pinoresinol diglucoside produced by Phomopsis sp. isolated from Eucommia ulmoides Oliv.[Pubmed: 22048615]Pinoresinol diglucoside (PDG) is the important antihypertensive compound in Eucommia ulmoides Oliv., a traditional Chinese herb medicine. The research objective was to certify the possibility of producing PDG through fermentation.
|

Pinoresinol diglucoside Dilution Calculator

Pinoresinol diglucoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4648 mL | 7.3242 mL | 14.6484 mL | 29.2967 mL | 36.6209 mL |
| 5 mM | 0.293 mL | 1.4648 mL | 2.9297 mL | 5.8593 mL | 7.3242 mL |
| 10 mM | 0.1465 mL | 0.7324 mL | 1.4648 mL | 2.9297 mL | 3.6621 mL |
| 50 mM | 0.0293 mL | 0.1465 mL | 0.293 mL | 0.5859 mL | 0.7324 mL |
| 100 mM | 0.0146 mL | 0.0732 mL | 0.1465 mL | 0.293 mL | 0.3662 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pinoresinol Diglucoside is one of the major lignans with various pharmacological activities which could be isolated from Duzhong and other plant species.
References:
[1]. Song JZ, et al. Development and validation of an ultra high-performance liquid chromatographic method for the determination of a diastereomeric impurity in (+)-pinoresinol diglucoside chemical reference substance. J Sep Sci. 2010 Jul;33(13):1909-15.
[2]. Zhang Y, et al. Production of pinoresinol diglucoside, pinoresinol monoglucoside, and pinoresinol by Phomopsis sp. XP-8 using mung bean and its major components. Appl Microbiol Biotechnol. 2015 Mar 25.
[3]. Shi J, et al. Structure identification and fermentation characteristics of pinoresinol diglucoside produced by Phomopsis sp. isolated from Eucommia ulmoides Oliv. Appl Microbiol Biotechnol. 2012 Feb;93(4):1475-83.
- Akuammidine
Catalog No.:BCN6509
CAS No.:639-36-1
- Ajmalidine
Catalog No.:BCN3491
CAS No.:639-30-5
- Gypsogenin
Catalog No.:BCC8993
CAS No.:639-14-5
- Cloxyfonac
Catalog No.:BCC5473
CAS No.:6386-63-6
- Meclofenamate Sodium
Catalog No.:BCC5490
CAS No.:6385-02-0
- NMDA (N-Methyl-D-aspartic acid)
Catalog No.:BCC4590
CAS No.:6384-92-5
- H-Glu-OMe
Catalog No.:BCC2924
CAS No.:6384-08-3
- Phyllostadimer A
Catalog No.:BCN4178
CAS No.:638203-32-4
- Z-D-Arg-OH
Catalog No.:BCC3575
CAS No.:6382-93-0
- 5-(3-Chlorophenyl)-N-[4-(morpholin-4-ylmethyl)phenyl]furan-2-carboxamide
Catalog No.:BCC3636
CAS No.:638156-11-3
- Dihydroalpinumisoflavone
Catalog No.:BCN4177
CAS No.:63807-90-9
- Erythrinin A
Catalog No.:BCN3203
CAS No.:63807-86-3
- Zinc Phytate
Catalog No.:BCN8302
CAS No.:63903-51-5
- LH846
Catalog No.:BCC4246
CAS No.:639052-78-1
- VUF 5681 dihydrobromide
Catalog No.:BCC7383
CAS No.:639089-06-8
- VX-680 (MK-0457,Tozasertib)
Catalog No.:BCC2167
CAS No.:639089-54-6
- (3R,10S)-Heptadeca-1,8-diene-4,6-diyne-3,10-diol
Catalog No.:BCC9111
CAS No.:63910-76-9
- Artemisinin
Catalog No.:BCN5814
CAS No.:63968-64-9
- Hopeyhopin
Catalog No.:BCN7533
CAS No.:63975-56-4
- 13-Oxopodocarp-8(14)-en-18-oic acid
Catalog No.:BCN4009
CAS No.:63976-69-2
- Lobeline Hydrochloride
Catalog No.:BCC8202
CAS No.:63990-84-1
- Physostigmine hemisulfate
Catalog No.:BCC6724
CAS No.:64-47-1
- Demeclocycline hydrochloride
Catalog No.:BCC5303
CAS No.:64-73-3
- Tetracycline Hydrochloride
Catalog No.:BCC1206
CAS No.:64-75-5
Heat shock factor 1 inducers from the bark of Eucommia ulmoides as cytoprotective agents.[Pubmed:23847077]
Chem Biodivers. 2013 Jul;10(7):1322-7.
The barks of Eucommia ulmoides (Eucommiae Cortex, Eucommiaceae) have been used as a traditional medicine in Korea, Japan, and China to treat hypertension, reinforce the muscles and bones, and recover the damaged liver and kidney functions. Among these traditional uses, to establish the recovery effects on the damaged organs on the basis of phytochemistry, the barks of E. ulmoides have been investigated to afford three known phenolic compounds, coniferaldehyde glucoside (1), bartsioside (2), and feretoside (3), which were found in the family Eucommiaceae for the first time. The compounds 1-3 were evaluated for their inducible activities on the heat shock factor 1 (HSF1), and heat shock proteins (HSPs) 27 and 70, along with four compounds, geniposide (4), geniposidic acid (5), Pinoresinol diglucoside (6), and liriodendrin (7), which were previously reported from E. ulmoides. Compounds 1-7 increased expression of HSF1 by a factor of 1.214, 1.144, 1.153, 1.114, 1.159, 1.041, and 1.167 at 3 muM, respectively. Coniferaldehyde glucoside (1) showed the most effective increase of HSF1 and induced successive expressions of HSP27 and HSP70 in a dose-dependent manner without cellular cytotoxicity, suggesting a possible application as a HSP inducer to act as cytoprotective agent.
Structure identification and fermentation characteristics of pinoresinol diglucoside produced by Phomopsis sp. isolated from Eucommia ulmoides Oliv.[Pubmed:22048615]
Appl Microbiol Biotechnol. 2012 Feb;93(4):1475-83.
Pinoresinol diglucoside (PDG) is the important antihypertensive compound in Eucommia ulmoides Oliv., a traditional Chinese herb medicine. The research objective was to certify the possibility of producing PDG through fermentation. PDG-producing endophytic fungi were isolated from E. ulmoides Oliv., and the highest PDG-yielding (11.65 mg/L) isolate, XP-8, was identified as Phomopsis sp. according to the morphological characteristics and the phylogenetic tree constructed on the basis of the gene sequence in the internal transcribed spacers district. The microbial PDG was isolated by using S-8 resin and purified to a purity of 98.7% using preparative high-performance liquid chromatography (HPLC). Information obtained from the UV spectrum (277 and 227 nm, in water solution), infra-red spectrum (3,428; 2,930; 2,877; 1,637; 1,600; and 1,513; 1,460; 1,421; 1,269; 1,223; 1,075; 658 cm(-1), in powder), molecular weight (682 Da, measured using HPLC-electrospray ionization mass spectrometry (ESI/MS) and tandem mass spectrometry), and nuclear magnetic resonance analysis show the microbial PDG is (+)-1-pinoresinol 4,4'-di-O-beta-D-glucopyranoside, same as the plant-derived PDG. The microbial PDG is stable in pH range from 3 to 11 but less stable at temperature higher than 90 degrees C and in light exposure. During the fermentation, PDG production outside cells starts at the later stage of cell growth when the residual sugar in the medium was low. The study reveals the possibility for production of PDG by fermentation.
Biotransformation of pinoresinol diglucoside to mammalian lignans by human intestinal microflora, and isolation of Enterococcus faecalis strain PDG-1 responsible for the transformation of (+)-pinoresinol to (+)-lariciresinol.[Pubmed:12736449]
Chem Pharm Bull (Tokyo). 2003 May;51(5):508-15.
By anaerobic incubation of Pinoresinol diglucoside (1) from the bark of Eucommia ulmoides with a fecal suspension of humans, eleven metabolites were formed, and their structures were identified as (+)-pinoresinol (2), (+)-lariciresinol (3), 3'-demethyl-(+)-lariciresinol (4), (-)-secoisolariciresinol (5), (-)-3-(3", 4"-dihydroxybenzyl)-2-(4'-hydroxy-3'-methoxybenzyl)butane-1, 4-diol (6), 2-(3', 4'-dihydroxybenzyl)-3-(3", 4"-dihydroxybenzyl)butane-1, 4-diol (7), 3-(3"-hydroxybenzyl)-2-(4'-hydroxy-3'-methoxybenzyl)butane-1, 4-diol (8), 2-(3', 4'-dihydroxybenzyl)-3-(3"-hydroxybenzyl)butane-1, 4-diol (9), (-)-enterodiol (10), (-)-(2R, 3R)-3-(3", 4"-dihydroxybenzyl)-2-(4'-hydroxy-3'-methoxybenzyl)butyrolactone (11), (-)-(2R, 3R)-2-(3', 4'-dihydroxybenzyl)-3-(3", 4"-dihydroxybenzyl)butyrolactone (12), (-)-(2R, 3R)-3-(3"-hydroxybenzyl)-2-(4'-hydroxy-3'-methoxybenzyl)butyrolactone (13), 2-(3', 4'-dihydroxybenzyl)-3-(3"-hydroxybenzyl)butyrolactone (14), 2-(3'-hydroxybenzyl)-3-(3", 4"-dihydroxybenzyl)butyrolactone (15) and (-)-(2R, 3R)-enterolactone (16) by various spectroscopic means, including two dimensional (2D)-NMR, mass spectrometry and circular dichroism. A possible metabolic pathway was proposed on the basis of their structures and time course experiments monitored by thin-layer chromatography. Furthermore, a bacterial strain responsible for the transformation of (+)-pinoresinol to (+)-lariciresinol was isolated from a human fecal suspension and identified as Enterococcus faecalis strain PDG-1.


