Lobeline HydrochlorideCAS# 63990-84-1 |
- (-)-Lobeline hydrochloride
Catalog No.:BCC6927
CAS No.:134-63-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 63990-84-1 | SDF | Download SDF |
| PubChem ID | 46202 | Appearance | Powder |
| Formula | C22H28ClNO2 | M.Wt | 373.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-[6-(2-hydroxy-2-phenylethyl)-1-methylpiperidin-2-yl]-1-phenylethanone;hydrochloride | ||
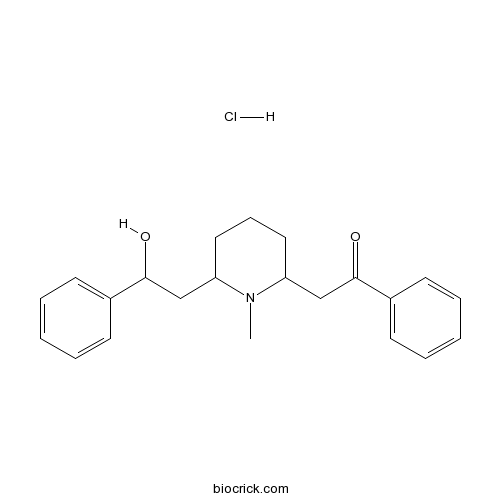
| SMILES | CN1C(CCCC1CC(=O)C2=CC=CC=C2)CC(C3=CC=CC=C3)O.Cl | ||
| Standard InChIKey | MKMYPTLXLWOUSO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H27NO2.ClH/c1-23-19(15-21(24)17-9-4-2-5-10-17)13-8-14-20(23)16-22(25)18-11-6-3-7-12-18;/h2-7,9-12,19-21,24H,8,13-16H2,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Lobeline Hydrochloride Dilution Calculator

Lobeline Hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6745 mL | 13.3726 mL | 26.7451 mL | 53.4902 mL | 66.8628 mL |
| 5 mM | 0.5349 mL | 2.6745 mL | 5.349 mL | 10.698 mL | 13.3726 mL |
| 10 mM | 0.2675 mL | 1.3373 mL | 2.6745 mL | 5.349 mL | 6.6863 mL |
| 50 mM | 0.0535 mL | 0.2675 mL | 0.5349 mL | 1.0698 mL | 1.3373 mL |
| 100 mM | 0.0267 mL | 0.1337 mL | 0.2675 mL | 0.5349 mL | 0.6686 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 13-Oxopodocarp-8(14)-en-18-oic acid
Catalog No.:BCN4009
CAS No.:63976-69-2
- Hopeyhopin
Catalog No.:BCN7533
CAS No.:63975-56-4
- Artemisinin
Catalog No.:BCN5814
CAS No.:63968-64-9
- (3R,10S)-Heptadeca-1,8-diene-4,6-diyne-3,10-diol
Catalog No.:BCC9111
CAS No.:63910-76-9
- VX-680 (MK-0457,Tozasertib)
Catalog No.:BCC2167
CAS No.:639089-54-6
- VUF 5681 dihydrobromide
Catalog No.:BCC7383
CAS No.:639089-06-8
- LH846
Catalog No.:BCC4246
CAS No.:639052-78-1
- Zinc Phytate
Catalog No.:BCN8302
CAS No.:63903-51-5
- Pinoresinol diglucoside
Catalog No.:BCN1093
CAS No.:63902-38-5
- Akuammidine
Catalog No.:BCN6509
CAS No.:639-36-1
- Ajmalidine
Catalog No.:BCN3491
CAS No.:639-30-5
- Gypsogenin
Catalog No.:BCC8993
CAS No.:639-14-5
- Physostigmine hemisulfate
Catalog No.:BCC6724
CAS No.:64-47-1
- Demeclocycline hydrochloride
Catalog No.:BCC5303
CAS No.:64-73-3
- Tetracycline Hydrochloride
Catalog No.:BCC1206
CAS No.:64-75-5
- Tolbutamide
Catalog No.:BCC5001
CAS No.:64-77-7
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- Etimizol
Catalog No.:BCC1562
CAS No.:64-99-3
- H-D-Val-OH
Catalog No.:BCC3145
CAS No.:640-68-6
- Paroxetine maleate
Catalog No.:BCC7265
CAS No.:64006-44-6
- Tamgermanetin
Catalog No.:BCN7944
CAS No.:640235-90-1
- 4(15)-Oppositene-1,7-diol
Catalog No.:BCN4180
CAS No.:640289-58-3
- Torachrysone 8-O-glucoside
Catalog No.:BCN4181
CAS No.:64032-49-1
- Boc-Lys(Ac)-OH
Catalog No.:BCC3411
CAS No.:6404-26-8
Surface plasmon resonance based biosensors for exploring the influence of alkaloids on aggregation of amyloid-beta peptide.[Pubmed:22163834]
Sensors (Basel). 2011;11(4):4030-42.
The main objective of the presented study was the development of a simple analytical tool for exploring the influence of naturally occurring compounds on the aggregation of amyloid-beta peptide (Abeta(40)) in order to find potential anti-neurodegenerative drugs. The gold discs used for surface plasmon resonance (SPR) measurements were modified with thioaliphatic acid. The surface functionalized with carboxylic groups was used for covalent attaching of Abeta(40) probe by creation of amide bonds in the presence of EDC/NHS. The modified SPR gold discs were used for exploring the Abeta(40) aggregation process in the presence of selected alkaloids: arecoline hydrobromide, pseudopelletierine hydrochloride, trigonelline hydrochloride and alpha-Lobeline Hydrochloride. The obtained results were discussed with other parameters which govern the phenomenon studied such as lipophilicity/hydrophilicy and Abeta(40)-alkaloid association constants.
Endoscopic scoring of the tracheal septum in horses and its clinical relevance for the evaluation of lower airway health in horses.[Pubmed:17378438]
Equine Vet J. 2007 Mar;39(2):107-12.
REASONS FOR PERFORMING STUDY: Although endoscopic scoring of the tracheal septum thickness is used as a diagnostic tool for evaluation of lower airway disease, its clinical relevance and reliability have never been critically assessed in the horse. OBJECTIVES: To investigate if septum thickness scores (STS) are reliable and serve as a clinically useful indicator of lower airway disease status and/or inflammation. METHODS: The variance of STS attributable to the horse, observer and changes over time was determined. The distribution of STS in a population of clinically normal horses and correlations of STS with age, gender, as well as mucus accumulation and cell differentials of tracheobronchial secretions and bronchoalveolar lavage fluid were investigated. Effects of altered pulmonary ventilation, induced by different drugs, on STS were assessed. Finally, STS of horses affected with recurrent airway obstruction (RAO) were compared to those of clinically normal horses. RESULTS: Recorded STS showed excellent intra- and satisfactory interobserver agreement Established clinical, endoscopic and cytological measures of lower airway inflammation, i.e. mucus accumulation scores and airway neutrophilia, did not correlate with STS. In horses age > or = 10 years, septum scores were significantly higher (P = 0.022) than in younger horses. Septum thickness scores did not differ significantly between clinically normal and RAO-affected horses both in exacerbation and in remission. Horses with markedly increased breathing effort (i.e. with metacholine- or Lobeline Hydrochloride-challenge), often differed markedly (up to 1.9 scores), but the average of end-inspiratory and end-expiratory STS did not differ from baseline STS. CONCLUSIONS AND CLINICAL RELEVANCE: Endoscopic STS are a reproducible measure, but STS did not correlate with clinical, endoscopic and cytological findings indicative of RAO or inflammatory airway disease.
Effects of tension of the girth strap on respiratory system mechanics in horses at rest and during hyperpnea induced by administration of lobeline hydrochloride.[Pubmed:16111154]
Am J Vet Res. 2005 Jul;66(7):1167-74.
OBJECTIVE: To determine whether tension of the girth strap of a saddle would sufficiently affect rib motion and reduce lung volume to alter pulmonary resistance in horses. ANIMALS: 10 healthy adult horses. PROCEDURE: We used classical techniques to measure the effects of tightening a girth strap (15 kg of tension) on pulmonary dynamics during eupnea and hyperpnea in horses. Respiratory impedance was evaluated by use of oscillometry, and resistance and reactance data were partitioned into lung and chest wall components. Rib cage and abdominal contributions to tidal volume and minute ventilation were measured by use of respiratory inductance plethysmography. Effects of strap tension on functional residual capacity (FRC) were measured during eupnea by use of a helium-dilution technique. In a subgroup of 6 horses, we also measured transdiaphragmatic pressures during eupnea and hyperpnea induced by administration of Lobeline Hydrochloride (0.2 mg/kg, i.v.). RESULTS: Pulmonary resistance measured by use of oscillometry but not by use of classical methods was significantly increased by the tension of the girth strap. However, the increase in pulmonary resistance could not be explained by a decrease in FRC. Motion of the rib cage was significantly reduced during eupnea and hyperpnea. However, ventilatory variables (tidal volume, minute ventilation, and peak flows), FRC, and transdiaphragmatic pressures were unaltered by strap tension. CONCLUSIONS AND CLINICAL RELEVANCE: Although tension of the girth strap caused measurable changes in respiratory mechanics (loss of rib motion and increased pulmonary resistance), there was no evidence that ventilation was limited.
Influence of breathing pattern and lung inflation on impulse oscillometry measurements in horses.[Pubmed:15501143]
Vet J. 2004 Nov;168(3):259-69.
The objective of this paper was to determine if changes in ventilation patterns could influence the outcome of respiratory function measurements performed with our impulse oscillometry system (IOS) in horses. In a first study, IOS tests were performed in vitro on six isolated equine lungs. Lung inflation levels were controlled by modifying depressurisation inside an artificial thorax and different ventilation patterns were imposed. In a second in vivo study, transient variations in breathing pattern were evaluated both with the IOS and a current reference technique (CRT) in five healthy mature horses after an intravenous (i.v.) injection of Lobeline Hydrochloride. In both studies, respiratory rate (RR, range: 7-42 breaths/min.) and tidal volume (V(T), range: 0.4-25 L) had minor or no influence on IOS parameters. The influence of lung inflation, most marked for resistance at 5 Hz (R(5 Hz)), was limited for the considered physiological range. In vivo, statistical models indicated that maximal changes in pleural pressure (Max Delta Ppl) and peak flows were the main determinants of the variability of the resistance (R(rs)) and the reactance (X(rs)) of the respiratory system. The fourfold increase in baseline Max Delta Ppl and peak flows obtained during hyperpnoea caused a significant increase in R(rs) at 5 and 10 Hz and a decrease in X(rs) at all frequencies. We conclude that IOS parameters are not influenced by tachypnoea, but will reflect alterations in respiratory mechanics caused by hyperpnoeic breathing.
Ultrasound spirometry in the horse: a preliminary report on the method and the effects of xylazine and lobeline hydrochloride medication.[Pubmed:9451919]
Schweiz Arch Tierheilkd. 1997;139(12):558-63.
A new computerised ultrasound-based spirometry system according to Buess et al. (1995) modified by a double flow measurement facility was used to study pulmonary function in healthy horses and horses affected with subclinical and manifest chronic bronchiolitis (CB). The horses were first evaluated at rest without any medication. On another occasion all horses were tested following i.v. administration of xylazine (0.4 mg/kg) and following i.v. administration of Lobeline Hydrochloride (l.hy.; 0.2 mg/kg) to evaluate the effect of xylazine and l.hy. on different spirometric variables. Ultrasound-based spirometry proved to be an easily applicable method for lung function testing, even in difficult horses. However, there existed a pronounced physiological variation for all measured lung function parameters and no significant differences between healthy horses and horses with chronic bronchiolitis could be found except for the expiratory tidal volume (VTE p < 0.05). Individually, a marked decrease of variability from breath to breath following either xylazine and l.hy. administration could be observed for all parameters, except the flow-time-ratio (Tpef./ Texp.) and the flow-volume-ratio (Vpef./Vexp).


