XL335FXR agonist CAS# 629664-81-9 |
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- SCH-1473759
Catalog No.:BCC1934
CAS No.:1094069-99-4
- VX-680 (MK-0457,Tozasertib)
Catalog No.:BCC2167
CAS No.:639089-54-6
- Reversine
Catalog No.:BCC1892
CAS No.:656820-32-5
- AZD1152
Catalog No.:BCC1393
CAS No.:722543-31-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 629664-81-9 | SDF | Download SDF |
| PubChem ID | 10026128 | Appearance | Powder |
| Formula | C25H24F2N2O3 | M.Wt | 438.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | XL335; Turofexorate isopropyl | ||
| Solubility | DMSO : 25 mg/mL (57.02 mM; Need ultrasonic) | ||
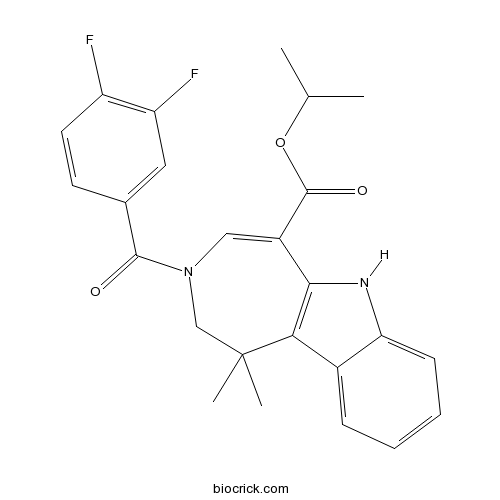
| Chemical Name | propan-2-yl 3-(3,4-difluorobenzoyl)-1,1-dimethyl-2,6-dihydroazepino[4,5-b]indole-5-carboxylate | ||
| SMILES | CC(C)OC(=O)C1=CN(CC(C2=C1NC3=CC=CC=C32)(C)C)C(=O)C4=CC(=C(C=C4)F)F | ||
| Standard InChIKey | INASOKQDNHHMRE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H24F2N2O3/c1-14(2)32-24(31)17-12-29(23(30)15-9-10-18(26)19(27)11-15)13-25(3,4)21-16-7-5-6-8-20(16)28-22(17)21/h5-12,14,28H,13H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | WAY-362450 is a potent, selective, and orally bioavailable FXR agonist with EC50 of 4 nM.In Vitro:WAY-362450 is a potent, selective, and orally bioavailable FXR agonist (EC50=4 nM). WAY-362450 is highly selective, as no significant cross-reactivity with these receptors (LXRα, LXRβ, PPARα, PPARγ, PPARδ, RXRα, RARγ, VDR, SXR, ERα, ERβ, GR, AR, MR, and PR) is observed at concentrations up to 10 μM. WAY-362450 displays potent agonist activity in the FXR reporter gene assays and on FXR target genes in cell-based assays. In promoter assays, utilizing reporter constructs under control of the human bile salt excretory pump (BSEP), human small heterodimer partner (SHP), and mouse intestinal bile acid binding protein (IBABP) genes are up-regulated by WAY-362450 with EC50 of 17, 230, and 33 nM, respectively. In addition, WAY-362450 significantly induces mRNAs encoding for BSEP, SHP, and IBABP in human cell cultures at 1 μM (13-, 2-, and 20-fold, respectively)[1]. WAY-362450 potently induces luciferase reporter expression with an EC50 of 16 nM. WAY-362450 is a potent stimulator of endogenous FXR gene activation in mouse AML12 cells and in primary human hepatocytes[2].In Vivo:WAY-362450 also shows potent effects on cholesterol and triglyceride lowering in LDLR-/- mice and antiatherogenic activity with respect to reduction of aortic arch lesions. Oral administration of WAY-362450 to LDLR-/- mice results in lowering of cholesterol and triglycerides. Chronic administration in an atherosclerosis model results in significant reduction in aortic arch lesions. WAY-362450 is dosed in rat at 3 mg/kg (po and iv) and shows good oral bioavailability (38%). There is a protracted half-life of 25 h, modest volume of distribution, and low clearance (3.3 L/kg, ~10% of hepatic blood flow). Additional pharmacokinetic studies in mice and higher species have been completed, and the data will be reported elsewhere[1]. In rats, WAY-362450 results in an elevation in HDLc levels, whereas in hamsters it causes a reduction similar to that observed in mice[2] Treatment of wild-type mice with 30 mg/kg WAY-362450 results in induction of SHP expression in wild-type mice but not in FXR-/- mice. Consistent with the known effects of SHP induction on bile acid synthetic gene expression, WAY-362450 strongly represses expression of the CYP8B1 bile acid synthetic gene in wild-type mice but had no effect on CYP8B1 gene expression in FXR-/- mice[3]. References: | |||||

XL335 Dilution Calculator

XL335 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2806 mL | 11.403 mL | 22.8061 mL | 45.6121 mL | 57.0151 mL |
| 5 mM | 0.4561 mL | 2.2806 mL | 4.5612 mL | 9.1224 mL | 11.403 mL |
| 10 mM | 0.2281 mL | 1.1403 mL | 2.2806 mL | 4.5612 mL | 5.7015 mL |
| 50 mM | 0.0456 mL | 0.2281 mL | 0.4561 mL | 0.9122 mL | 1.1403 mL |
| 100 mM | 0.0228 mL | 0.114 mL | 0.2281 mL | 0.4561 mL | 0.5702 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
XL335 is a potent, selective and orally bioavailable agonist of the farnesoid X receptor (FXR) with EC50 value of 4 nM [1].
XL335 has shown to reduce IL-6-induced both mRNA and protein expression of CRP via FXR in human hepatoma Hep3B cells. XL335 remarkably reduced LPS-induced SAP and SAA3 mRNA expression in WT mice, but not in FXR/KO mice [2].
Additionally, in hepatoma cells, XL335 block the lipid accumulation induced by palmitic acid (PA). In vivo, XL335 has shown to decrease portal vein endotoxin level and reduce inflammation induced by fructose in mice. XL335 attenuated inflammation and suppressed ADRP expression in lipopolysaccharide (LPS)-induced mice [3].
References:
[1] Flatt B1, Martin R, Wang TL, Mahaney P, Murphy B, Gu XH, Foster P, Li J, Pircher P, Petrowski M, Schulman I, Westin S, Wrobel J, Yan G, Bischoff E, Daige C,Mohan R. Discovery of XL335 (WAY-362450), a highly potent, selective, and orally active agonist of the farnesoid X receptor (FXR). J Med Chem. 2009 Feb 26;52(4):904-7. doi: 10.1021/jm8014124.
[2] Zhang S1, Liu Q, Wang J, Harnish DC. Suppression of interleukin-6-induced C-reactive protein expression by FXR agonists. Biochem Biophys Res Commun. 2009 Feb 6;379(2):476-9.
[3] Liu X1, Xue R2, Ji L1, Zhang X1, Wu J3, Gu J1, Zhou M4, Chen S5. Activation of farnesoid X receptor (FXR) protects against fructose-induced liver steatosis via inflammatory inhibition and ADRP reduction. Biochem Biophys Res Commun. 2014 Jul 18;450(1):117-23.
- Boc-Tle-OH
Catalog No.:BCC3343
CAS No.:62965-35-9
- Gnetucleistol D
Catalog No.:BCN3400
CAS No.:629643-26-1
- Gomisin G
Catalog No.:BCN2269
CAS No.:62956-48-3
- Gomisin F
Catalog No.:BCN3625
CAS No.:62956-47-2
- (2-Benzothiazolylthio)acetic acid
Catalog No.:BCC8387
CAS No.:6295-57-4
- 6-Methoxy-4-methylcoumarin
Catalog No.:BCN6537
CAS No.:6295-35-8
- Morusinol
Catalog No.:BCN4168
CAS No.:62949-93-3
- Mulberrin
Catalog No.:BCN4167
CAS No.:62949-79-5
- Kuwanon A
Catalog No.:BCN2944
CAS No.:62949-77-3
- H-D-Pro-NH2
Catalog No.:BCC3024
CAS No.:62937-45-5
- Procaterol hydrochloride
Catalog No.:BCC6937
CAS No.:62929-91-3
- Heptanal oxime
Catalog No.:BCN2267
CAS No.:629-31-2
- 6-Aminoquinoxaline
Catalog No.:BCC8767
CAS No.:6298-37-9
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
- Androstenedione
Catalog No.:BCC8296
CAS No.:63-05-8
- Primaquine Diphosphate
Catalog No.:BCC4706
CAS No.:63-45-6
- H-Met-OH
Catalog No.:BCC2993
CAS No.:63-68-3
- Sulfanilamide
Catalog No.:BCC4858
CAS No.:63-74-1
- L-Phenylalanine
Catalog No.:BCN3818
CAS No.:63-91-2
- Phenoxybenzamine HCl
Catalog No.:BCC4334
CAS No.:63-92-3
- Nonacosane
Catalog No.:BCC9102
CAS No.:630-03-5
- Ouabain
Catalog No.:BCC5069
CAS No.:630-60-4
- Phenytoin sodium
Catalog No.:BCC5071
CAS No.:630-93-3
- Corynoxeine
Catalog No.:BCN5002
CAS No.:630-94-4
Discovery of XL335 (WAY-362450), a highly potent, selective, and orally active agonist of the farnesoid X receptor (FXR).[Pubmed:19159286]
J Med Chem. 2009 Feb 26;52(4):904-7.
Azepino[4,5-b]indoles have been identified as potent agonists of the farnesoid X receptor (FXR). In vitro and in vivo optimization has led to the discovery of 6m (XL335, WAY-362450) as a potent, selective, and orally bioavailable FXR agonist (EC(50) = 4 nM, Eff = 149%). Oral administration of 6m to LDLR(-/-) mice results in lowering of cholesterol and triglycerides. Chronic administration in an atherosclerosis model results in significant reduction in aortic arch lesions.


