Gomisin GCAS# 62956-48-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 62956-48-3 | SDF | Download SDF |
| PubChem ID | 14992067 | Appearance | White powder |
| Formula | C30H32O9 | M.Wt | 536.6 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (93.18 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
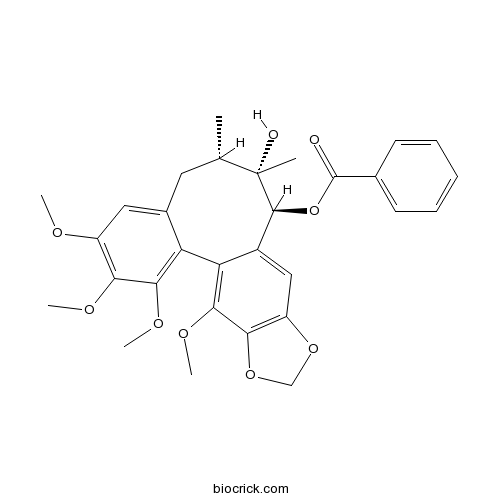
| SMILES | CC1CC2=CC(=C(C(=C2C3=C(C4=C(C=C3C(C1(C)O)OC(=O)C5=CC=CC=C5)OCO4)OC)OC)OC)OC | ||
| Standard InChIKey | OFDWKHIQKPKRKY-DSASHONVSA-N | ||
| Standard InChI | InChI=1S/C30H32O9/c1-16-12-18-13-20(33-3)24(34-4)26(35-5)22(18)23-19(14-21-25(27(23)36-6)38-15-37-21)28(30(16,2)32)39-29(31)17-10-8-7-9-11-17/h7-11,13-14,16,28,32H,12,15H2,1-6H3/t16-,28-,30-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gomisin G is a drug candidate for treatment of cardiovascular disease, and it is a good substrate of CYP2C9, and can be easily affected by the inhibitors of CYP2C9. Gomisin G exhibits anti-tumor activities, it exhibits potent anti-HIV activity with EC50 and therapeutic index (TI) values of 0.006 microgram/mL and 300, respectively. |
| Targets | HIV | P450 (e.g. CYP17) |
| In vitro | Anti-AIDS agents--XXVI. Structure-activity correlations of gomisin-G-related anti-HIV lignans from Kadsura interior and of related synthetic analogues.[Pubmed: 9313872]Bioorg Med Chem. 1997 Aug;5(8):1715-23.Bioactivity-directed fractionation of an ethanolic extract of the stems of Kadsura interior led to the isolation and identification of 12 known lignans (1-12).
In silico application in the prediction of herb-drug interaction for cerebrovascular diseases herbs[Reference: WebLink]Lat. Am. J. Pharm., 2016,35 (1): 192-4.
Herb-drug interaction remains to be a key factor limiting the clinical application of drugs and herbs. |
| Kinase Assay | Inhibition of human CYP3A4 and CYP3A5 enzymes by gomisin C and gomisin G, two lignan analogs derived from Schisandra chinensis.[Pubmed: 28344076 ]Drug-drug interation prediction between ketoconazole and anti-liver cancer drug Gomisin G.[Pubmed: 26124807 ]Afr Health Sci. 2015 Jun;15(2):590-3.Gomisin G, isolated from herb Schisandra chinensis, exhibits anti-tumor activities. Therefore, Gomisin G is a drug candidate for anti-liver cancer therapy.
To predict the metabolic behavior and metabolism-based drug-drug interaction of Gomisin G.
Fitoterapia. 2017 Jun;119:26-31.Gomisin C (GC) and Gomisin G (GG) are two lignan analogs isolated from the Traditional Chinese Medicine Schisandra chinensis which possesses multiple pharmacological activities. However, the potential herb-drug interactions (HDI) between these lignans and other drugs through inhibiting human cytochrome P450 3A4 (CYP3A4) and CYP3A5 remains unclear. |

Gomisin G Dilution Calculator

Gomisin G Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8636 mL | 9.3179 mL | 18.6359 mL | 37.2717 mL | 46.5896 mL |
| 5 mM | 0.3727 mL | 1.8636 mL | 3.7272 mL | 7.4543 mL | 9.3179 mL |
| 10 mM | 0.1864 mL | 0.9318 mL | 1.8636 mL | 3.7272 mL | 4.659 mL |
| 50 mM | 0.0373 mL | 0.1864 mL | 0.3727 mL | 0.7454 mL | 0.9318 mL |
| 100 mM | 0.0186 mL | 0.0932 mL | 0.1864 mL | 0.3727 mL | 0.4659 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Gomisin G is an ethanolic extract of the stems of Kadsura interior; exhibits potent anti-HIV activity with EC50 and therapeutic index (TI) values of 0.006 microgram/mL and 300, respectively.
References:
[1]. Chen DF, et al. Anti-AIDS agents--XXVI. Structure-activity correlations of gomisin-G-related anti-HIV lignans from Kadsura interior and of related synthetic analogues. Bioorg Med Chem. 1997 Aug;5(8):1715-23.
[2]. Ikeya Y, et al. The constituents of Schizandra chinensis Baill. I. Isolation and structure determination of five new lignans, gomisin A, B, C, F and G, and the absolute structure of schizandrin. Chem Pharm Bull (Tokyo). 1979 Jun;27(6):1383-94.
- Gomisin F
Catalog No.:BCN3625
CAS No.:62956-47-2
- (2-Benzothiazolylthio)acetic acid
Catalog No.:BCC8387
CAS No.:6295-57-4
- 6-Methoxy-4-methylcoumarin
Catalog No.:BCN6537
CAS No.:6295-35-8
- Morusinol
Catalog No.:BCN4168
CAS No.:62949-93-3
- Mulberrin
Catalog No.:BCN4167
CAS No.:62949-79-5
- Kuwanon A
Catalog No.:BCN2944
CAS No.:62949-77-3
- H-D-Pro-NH2
Catalog No.:BCC3024
CAS No.:62937-45-5
- Procaterol hydrochloride
Catalog No.:BCC6937
CAS No.:62929-91-3
- Heptanal oxime
Catalog No.:BCN2267
CAS No.:629-31-2
- Cefoperazone
Catalog No.:BCC3748
CAS No.:62893-19-0
- Hecubine
Catalog No.:BCN7467
CAS No.:62874-52-6
- TP 003
Catalog No.:BCC6169
CAS No.:628690-75-5
- Gnetucleistol D
Catalog No.:BCN3400
CAS No.:629643-26-1
- Boc-Tle-OH
Catalog No.:BCC3343
CAS No.:62965-35-9
- XL335
Catalog No.:BCC4501
CAS No.:629664-81-9
- 6-Aminoquinoxaline
Catalog No.:BCC8767
CAS No.:6298-37-9
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
- Androstenedione
Catalog No.:BCC8296
CAS No.:63-05-8
- Primaquine Diphosphate
Catalog No.:BCC4706
CAS No.:63-45-6
- H-Met-OH
Catalog No.:BCC2993
CAS No.:63-68-3
- Sulfanilamide
Catalog No.:BCC4858
CAS No.:63-74-1
- L-Phenylalanine
Catalog No.:BCN3818
CAS No.:63-91-2
- Phenoxybenzamine HCl
Catalog No.:BCC4334
CAS No.:63-92-3
- Nonacosane
Catalog No.:BCC9102
CAS No.:630-03-5
Inhibition of human CYP3A4 and CYP3A5 enzymes by gomisin C and gomisin G, two lignan analogs derived from Schisandra chinensis.[Pubmed:28344076]
Fitoterapia. 2017 Jun;119:26-31.
Gomisin C (GC) and Gomisin G (GG) are two lignan analogs isolated from the Traditional Chinese Medicine Schisandra chinensis which possesses multiple pharmacological activities. However, the potential herb-drug interactions (HDI) between these lignans and other drugs through inhibiting human cytochrome P450 3A4 (CYP3A4) and CYP3A5 remains unclear. In the present study, the inhibitory action of GC and GG on CYP3A4 and CYP3A5 were investigated. The results demonstrated that both GC and GG strongly inhibited CYP3A-mediated midazolam 1'-hydroxylation, nifedipine oxidation and testosterone 6beta-hydroxylation. Notably, the inhibitory intensity of GC towards CYP3A4 was stronger than CYP3A5 when using midazolam and nifedipine as substrates. While inhibition of GC towards CYP3A5 was weaker than CYP3A4 when using testosterone as substrate. In contrast, GG showed a stronger inhibitory activity on CYP3A5 than CYP3A4 without substrate-dependent behavior. In addition, docking simulations indicated that the pi-pi interaction between CYP3A4 and GC, and hydrogen-bond interaction between CYP3A5 and GG might result in their different inhibitory actions. Furthermore, the AUC of drugs metabolized by CYP3A was estimated to increase by 8%-321% and 2%-3190% in the presence of GC and GG, respectively. These findings strongly suggested that GC and GG showed high HDI potentials, and the position of methylenedioxy group determined their different inhibitory effect towards CYP3A4 and CYP3A5, which are of significance for the application of Schisandra chinensis-containing herbs.
Anti-AIDS agents--XXVI. Structure-activity correlations of gomisin-G-related anti-HIV lignans from Kadsura interior and of related synthetic analogues.[Pubmed:9313872]
Bioorg Med Chem. 1997 Aug;5(8):1715-23.
Bioactivity-directed fractionation of an ethanolic extract of the stems of Kadsura interior led to the isolation and identification of 12 known lignans (1-12). Seven of these compounds (1, 6, 8-12) were active as anti-HIV agents. Gomisin-G (11) exhibited the most potent anti-HIV activity with EC50 and therapeutic index (TI) values of 0.006 microgram/mL and 300, respectively. Schisantherin-D (6), kadsuranin (8), and schisandrin-C (10) showed good activity with EC50 values of 0.5, 0.8, and 1.2 micrograms/mL, and TI values of 110, 56, and 33.3, respectively. Ten related synthetic biphenyl compounds, five variously substituted bismethylenedioxy, dimethoxy, and dimethoxycarbonyl isomers (18-22) and five brominated derivatives (23-27) also were evaluated for inhibitory activity against HIV-1 replication in acutely infected H9 cells. The total syntheses of two new isomers (21 and 22) are reported for the first time. The anti-HIV data indicated that the relative position and types of substituents on the phenolic hydroxy groups of either the natural lignans or the synthetic biphenyl compounds rather than the numbers of bromine(s) on the aromatic rings are of primary importance. In the cyclooctane ring of the natural lignans, the position and substitution of hydroxy groups are also important to enhanced anti-HIV activity.


