6-Methoxy-4-methylcoumarinCAS# 6295-35-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6295-35-8 | SDF | Download SDF |
| PubChem ID | 223821 | Appearance | Powder |
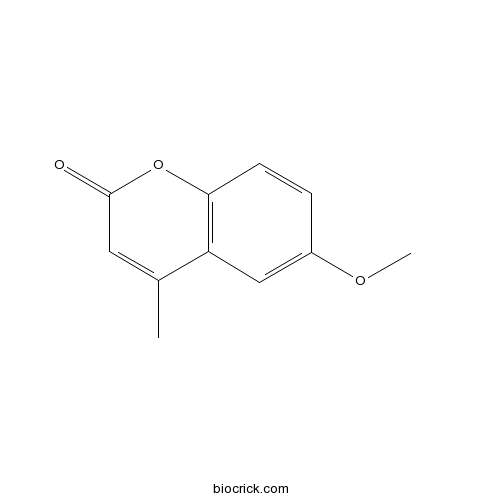
| Formula | C11H10O3 | M.Wt | 190.20 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 6-methoxy-4-methylchromen-2-one | ||
| SMILES | CC1=CC(=O)OC2=C1C=C(C=C2)OC | ||
| Standard InChIKey | KNGPIBWCWOQBEK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H10O3/c1-7-5-11(12)14-10-4-3-8(13-2)6-9(7)10/h3-6H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 6-Methoxy-4-methylcoumarin is a natural product from Eupatorium pauciflorum. |
| Structure Identification | Journal of Molecular Structure, 2014 , 1061 (1) :175-80.Study of dipole moments of some coumarin derivatives.[Reference: WebLink]
In this paper we report the dipole moments of two coumarin derivatives viz 6-Methoxy-4-methylcoumarin (6MMC) and 6-hydroxy-4-methylcoumarin (6HMC).
|

6-Methoxy-4-methylcoumarin Dilution Calculator

6-Methoxy-4-methylcoumarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2576 mL | 26.2881 mL | 52.5762 mL | 105.1525 mL | 131.4406 mL |
| 5 mM | 1.0515 mL | 5.2576 mL | 10.5152 mL | 21.0305 mL | 26.2881 mL |
| 10 mM | 0.5258 mL | 2.6288 mL | 5.2576 mL | 10.5152 mL | 13.1441 mL |
| 50 mM | 0.1052 mL | 0.5258 mL | 1.0515 mL | 2.103 mL | 2.6288 mL |
| 100 mM | 0.0526 mL | 0.2629 mL | 0.5258 mL | 1.0515 mL | 1.3144 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Morusinol
Catalog No.:BCN4168
CAS No.:62949-93-3
- Mulberrin
Catalog No.:BCN4167
CAS No.:62949-79-5
- Kuwanon A
Catalog No.:BCN2944
CAS No.:62949-77-3
- H-D-Pro-NH2
Catalog No.:BCC3024
CAS No.:62937-45-5
- Procaterol hydrochloride
Catalog No.:BCC6937
CAS No.:62929-91-3
- Heptanal oxime
Catalog No.:BCN2267
CAS No.:629-31-2
- Cefoperazone
Catalog No.:BCC3748
CAS No.:62893-19-0
- Hecubine
Catalog No.:BCN7467
CAS No.:62874-52-6
- TP 003
Catalog No.:BCC6169
CAS No.:628690-75-5
- Senampeline B
Catalog No.:BCN2031
CAS No.:62860-52-0
- 2-Amino-6-nitrobenzothiazole
Catalog No.:BCC8544
CAS No.:6285-57-0
- Senampeline E
Catalog No.:BCN2032
CAS No.:71075-42-8
- (2-Benzothiazolylthio)acetic acid
Catalog No.:BCC8387
CAS No.:6295-57-4
- Gomisin F
Catalog No.:BCN3625
CAS No.:62956-47-2
- Gomisin G
Catalog No.:BCN2269
CAS No.:62956-48-3
- Gnetucleistol D
Catalog No.:BCN3400
CAS No.:629643-26-1
- Boc-Tle-OH
Catalog No.:BCC3343
CAS No.:62965-35-9
- XL335
Catalog No.:BCC4501
CAS No.:629664-81-9
- 6-Aminoquinoxaline
Catalog No.:BCC8767
CAS No.:6298-37-9
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
- Androstenedione
Catalog No.:BCC8296
CAS No.:63-05-8
- Primaquine Diphosphate
Catalog No.:BCC4706
CAS No.:63-45-6
- H-Met-OH
Catalog No.:BCC2993
CAS No.:63-68-3
- Sulfanilamide
Catalog No.:BCC4858
CAS No.:63-74-1
Biochemical and Proteomic Characterization of Recombinant Human alpha/beta Hydrolase Domain 6.[Pubmed:30696836]
Sci Rep. 2019 Jan 29;9(1):890.
Human alpha/beta hydrolase domain 6 (hABHD6) is an enzyme that hydrolyzes 2-arachidonoylglycerol (2-AG), a potent agonist at both cannabinoid CB1 and CB2 receptors. In vivo modulation of ABHD6 expression has been shown to have potential therapeutic applications, making the enzyme a promising drug target. However, the lack of structural information on hABHD6 limits the discovery and design of selective inhibitors. We have performed E. coli expression, purification and activity profiling screening of different hABHD6 constructs and identified a truncated variant without N-terminal transmembrane (TM) domain, hDelta29-3-ABHD6, as the most promising protein for further characterization. The elimination of the TM domain did not affect 2-AG or fluorogenic arachidonoyl, 7-hydroxy-6-Methoxy-4-methylcoumarin ester (AHMMCE) substrates hydrolysis, suggesting that the TM is not essential for enzyme catalytic activity. The hDelta29-3-ABHD6 variant was purified in a single step using Immobilized Metal Affinity Chromatography (IMAC), in-solution trypsin digested, and proteomically characterized by Matrix Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS). The N-terminal peptide without methionine was identified indicating on a post-translational modification of the recombinant protein. The mechanism of inhibition of hABHD6 with AM6701 and WWL70 covalent probes was elucidated based on MS analysis of trypsin digested hABHD6 following the Ligand Assisted Protein Structure (LAPS) approach. We identified the carbamylated peptides containing catalytic serine (Ser(148)) suggesting a selective carbamylation of the enzyme by AM6701 or WWL70 and confirming an essential role of this residue in catalysis. The ability to produce substantial quantities of functional, pure hABHD6 will aid in the downstream structural characterization, and development of potent, selective inhibitors.
Full mass spectrometric characterization of human monoacylglycerol lipase generated by large-scale expression and single-step purification.[Pubmed:18452279]
J Proteome Res. 2008 May;7(5):2158-64.
The serine hydrolase monoacylglycerol lipase (MGL) modulates endocannabinoid signaling in vivo by inactivating 2-arachidonoylglycerol (2-AG), the main endogenous agonist for central CB1 and peripheral CB2 cannabinoid receptors. To characterize this key endocannabinoid enzyme by mass spectrometry-based proteomics, we first overexpressed recombinant hexa-histidine-tagged human MGL (hMGL) in Escherichia coli and purified it in a single chromatographic step with high yield (approximately 30 mg/L). With 2-AG as substrate, hMGL displayed an apparent V max of 25 micromol/(microg min) and K m of 19.7 microM, an affinity for 2-AG similar to that of native rat-brain MGL (rMGL) (Km=33.6 microM). hMGL also demonstrated a comparable affinity (Km approximately 8-9 microM) for the novel fluorogenic substrate, arachidonoyl, 7-hydroxy-6-Methoxy-4-methylcoumarin ester (AHMMCE), in a sensitive, high-throughput fluorometric MGL assay. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) unequivocably demonstrated the mass (34,126 Da) and purity of this hMGL preparation. After in-solution tryptic digestion, hMGL full proteomic characterization was carried out, which showed (1) an absence of intramolecular disulfide bridges in the functional, recombinant enzyme and (2) the post-translational removal of the enzyme's N-terminal methionine. Availability of sufficient quantities of pure, well-characterized hMGL will enable further molecular and structural profiling of this key endocannabinoid-system enzyme.


