Daclatasvir (BMS-790052)HCV NS5A inhibitor CAS# 1214735-16-6 |
- Anguizole
Catalog No.:BCC1365
CAS No.:442666-98-0
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Balapiravir
Catalog No.:BCC1396
CAS No.:690270-29-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1214735-16-6 | SDF | Download SDF |
| PubChem ID | 25154714 | Appearance | Powder |
| Formula | C40H50N8O6 | M.Wt | 738.88 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
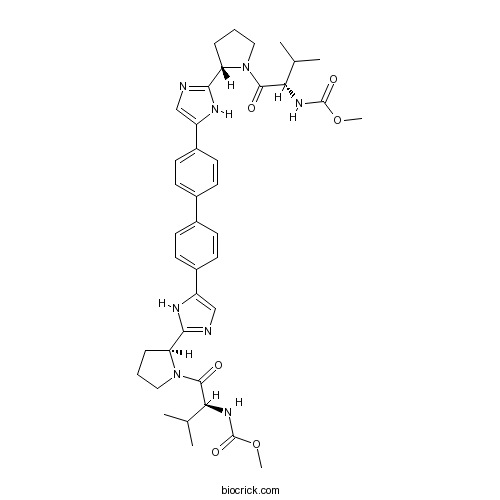
| Chemical Name | methyl N-[(2S)-1-[(2S)-2-[5-[4-[4-[2-[(2S)-1-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]pyrrolidin-2-yl]-1H-imidazol-5-yl]phenyl]phenyl]-1H-imidazol-2-yl]pyrrolidin-1-yl]-3-methyl-1-oxobutan-2-yl]carbamate | ||
| SMILES | CC(C)C(C(=O)N1CCCC1C2=NC=C(N2)C3=CC=C(C=C3)C4=CC=C(C=C4)C5=CN=C(N5)C6CCCN6C(=O)C(C(C)C)NC(=O)OC)NC(=O)OC | ||
| Standard InChIKey | FKRSSPOQAMALKA-CUPIEXAXSA-N | ||
| Standard InChI | InChI=1S/C40H50N8O6/c1-23(2)33(45-39(51)53-5)37(49)47-19-7-9-31(47)35-41-21-29(43-35)27-15-11-25(12-16-27)26-13-17-28(18-14-26)30-22-42-36(44-30)32-10-8-20-48(32)38(50)34(24(3)4)46-40(52)54-6/h11-18,21-24,31-34H,7-10,19-20H2,1-6H3,(H,41,43)(H,42,44)(H,45,51)(H,46,52)/t31-,32-,33-,34-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Daclatasvir (BMS-790052; EBP 883) is a first-in-class, highly-selective oral inhibitor of HCV NS5A with EC50 values of 9-50 pM. | |||||
| Targets | HCV NS5A | |||||
| IC50 | 9 pM-50 pM(EC50) | |||||

Daclatasvir (BMS-790052) Dilution Calculator

Daclatasvir (BMS-790052) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3534 mL | 6.767 mL | 13.534 mL | 27.068 mL | 33.835 mL |
| 5 mM | 0.2707 mL | 1.3534 mL | 2.7068 mL | 5.4136 mL | 6.767 mL |
| 10 mM | 0.1353 mL | 0.6767 mL | 1.3534 mL | 2.7068 mL | 3.3835 mL |
| 50 mM | 0.0271 mL | 0.1353 mL | 0.2707 mL | 0.5414 mL | 0.6767 mL |
| 100 mM | 0.0135 mL | 0.0677 mL | 0.1353 mL | 0.2707 mL | 0.3383 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Daclatasvir, formerly known as BMS-790052, is a potent NS5A inhibitor with EC50 values varying from 9 to 146 pM. [1,2]
The nonstructural 5A (NS5A) is a target in HCV drug development, which is a 447 amino-acid, zinc-binding phosphoprotein who plays an essential but enigmatic role in the virus life cycle. However the function of NS5A has no enzymatic activities, which makes it very difficult to understand the antiviral function of daclatasvir. It is assumed that Daclatasvir may interfere with the dimeric structure of NS5A, effecting subtle structural distortions that interfere with protein function in a specific way.[1,2]
The antiviral activity of daclatasvir towards genotypes was assessed by using replication-competent 1a or 1b replicons to construct hybrids in which the entire NS5A coding region or the first 100 amino acids of NS5A from different genotypes replaced the corresponding sequence of the parent replicon. Daclatasvir was reported to be highly potent across all HCV genotypes with half-maximum effective concentrations (EC50) ranging from 9 to 146 pM.[2]
A phase I clinical study showed Daclatasvir’s inhibition for HCV viruses. A 1 mg dose of daclatasvir produced a mean 1.8 log10 reduction in serum HCV RNA 24 h after administration. The 10 and 100 mg doses produced 3.2 log10 and 3.3 log10 reductions, respectively. Data collected from clinical trials on daclatasvir illustrated an initial, rapid viral decline followed by a slower fall in HCV RNA, which indicated that by inhibiting NS5A, daclatasvir blocks intracellular HCV RNA synthesis and virion assembly and secretion.[1]
References:
1.Herbst D A, Reddy K R. NS5A inhibitor, daclatasvir, for the treatment of chronic hepatitis C virus infection[J]. Expert opinion on investigational drugs, 2013, 22(10): 1337-1346.
2.Gao M, Nettles R E, Belema M, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect[J]. Nature, 2010, 465(7294): 96-100.
- Ajugapantin A
Catalog No.:BCN3663
CAS No.:121449-67-0
- WZ4003
Catalog No.:BCC4363
CAS No.:1214265-58-3
- WZ8040
Catalog No.:BCC1075
CAS No.:1214265-57-2
- WZ3146
Catalog No.:BCC4004
CAS No.:1214265-56-1
- POM 1
Catalog No.:BCC7454
CAS No.:12141-67-2
- N6-Benzyladenine
Catalog No.:BCC9076
CAS No.:1214-39-7
- Bernardioside A
Catalog No.:BCN7862
CAS No.:121368-52-3
- SR 33805 oxalate
Catalog No.:BCC7181
CAS No.:121346-33-6
- Fmoc-Glu-OH
Catalog No.:BCC3489
CAS No.:121343-82-6
- WZ4002
Catalog No.:BCC1074
CAS No.:1213269-23-8
- 1-Hydroxybisabola-2,10-dien-4-one
Catalog No.:BCN7297
CAS No.:1213251-45-6
- RWJ 21757
Catalog No.:BCC7460
CAS No.:121288-39-9
- 5-Benzyloxyindole
Catalog No.:BCC8742
CAS No.:1215-59-4
- PF-04991532
Catalog No.:BCC8094
CAS No.:1215197-37-7
- Salvianolic acid B; Lithospermic acid B; Danfensuan B
Catalog No.:BCC8249
CAS No.:121521-90-2
- SR 58611A hydrochloride
Catalog No.:BCC7833
CAS No.:121524-09-2
- GR 144053 trihydrochloride
Catalog No.:BCC6998
CAS No.:1215333-48-4
- RG2833
Catalog No.:BCC1893
CAS No.:1215493-56-3
- CFM 1571 hydrochloride
Catalog No.:BCC5924
CAS No.:1215548-30-3
- SB 242084
Catalog No.:BCC5949
CAS No.:1215566-78-1
- RS 100329 hydrochloride
Catalog No.:BCC5741
CAS No.:1215654-26-4
- CP-809101 hydrochloride
Catalog No.:BCC1499
CAS No.:1215721-40-6
- NBI 27914 hydrochloride
Catalog No.:BCC7124
CAS No.:1215766-76-9
- Gatifloxacin hydrochloride
Catalog No.:BCC4224
CAS No.:121577-32-0
Asymmetric binding to NS5A by daclatasvir (BMS-790052) and analogs suggests two novel modes of HCV inhibition.[Pubmed:25365735]
J Med Chem. 2014 Dec 11;57(23):10031-43.
Symmetric, dimeric Daclatasvir (BMS-790052) is the clinical lead for a class of picomolar inhibitors of HCV replication. While specific, resistance-bearing mutations at positions 31 and 93 of domain I strongly suggest the viral NS5A as target, structural mechanism(s) for the drugs' activities and resistance remains unclear. Several previous models suggested symmetric binding modes relative to the homodimeric target; however, none can fully explain SAR details for this class. We present semiautomated workflows to model potential receptor conformations for docking. Surprisingly, ranking docked hits with our library-derived 3D-pharmacophore revealed two distinct asymmetric binding modes, at a conserved poly-proline region between 31 and 93, consistent with SAR. Interfering with protein-protein interactions at this membrane interface can explain potent inhibition of replication-complex formation, resistance, effects on lipid droplet distribution, and virion release. These detailed interaction models and proposed mechanisms of action will allow structure-based design of new NS5A directed compounds with higher barriers to HCV resistance.


