CP-809101 hydrochloride5-HT2C receptor agonist, potent and selective CAS# 1215721-40-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1215721-40-6 | SDF | Download SDF |
| PubChem ID | 56972220 | Appearance | Powder |
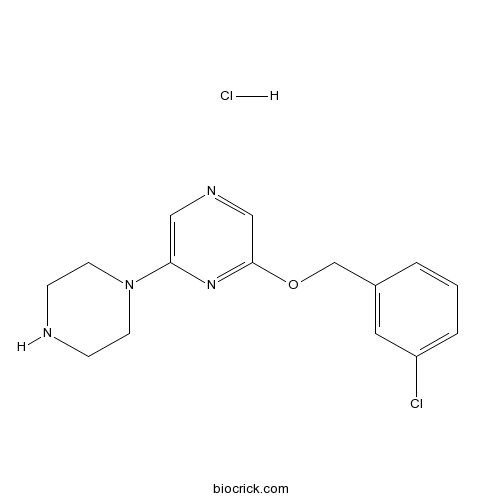
| Formula | C15H18Cl2N4O | M.Wt | 341.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 20 mg/mL (58.61 mM; Need ultrasonic) | ||
| Chemical Name | 2-[(3-chlorophenyl)methoxy]-6-piperazin-1-ylpyrazine;hydrochloride | ||
| SMILES | C1CN(CCN1)C2=CN=CC(=N2)OCC3=CC(=CC=C3)Cl.Cl | ||
| Standard InChIKey | NMUNRTCTDLORDR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H17ClN4O.ClH/c16-13-3-1-2-12(8-13)11-21-15-10-18-9-14(19-15)20-6-4-17-5-7-20;/h1-3,8-10,17H,4-7,11H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective 5-HT2C receptor agonist (pEC50 values are 9.96, 7.19 and 6.81 for human 5-HT2C, 5-HT2B and 5-HT2A receptors respectively). Displays antipsychotic activity; suppresses condition avoidance responding (CAR) and inhibits PCP and amphetamine-stimulated hyperactivity in rats following subcutaneous administration. |

CP-809101 hydrochloride Dilution Calculator

CP-809101 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9305 mL | 14.6524 mL | 29.3049 mL | 58.6098 mL | 73.2622 mL |
| 5 mM | 0.5861 mL | 2.9305 mL | 5.861 mL | 11.722 mL | 14.6524 mL |
| 10 mM | 0.293 mL | 1.4652 mL | 2.9305 mL | 5.861 mL | 7.3262 mL |
| 50 mM | 0.0586 mL | 0.293 mL | 0.5861 mL | 1.1722 mL | 1.4652 mL |
| 100 mM | 0.0293 mL | 0.1465 mL | 0.293 mL | 0.5861 mL | 0.7326 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CP-809101 is a potent and selective 5-HT2C receptor agonist (pEC50 values are 9.96, 7.19 and 6.81 for human 5-HT2C, 5-HT2B and 5-HT2A receptors respectively). CP-809101 displays antipsychotic activity; suppresses condition avoidance responding (CAR) and inhibits PCP and amphetamine-stimulated hyperactivity in rats following subcutaneous administration.
- RS 100329 hydrochloride
Catalog No.:BCC5741
CAS No.:1215654-26-4
- SB 242084
Catalog No.:BCC5949
CAS No.:1215566-78-1
- CFM 1571 hydrochloride
Catalog No.:BCC5924
CAS No.:1215548-30-3
- RG2833
Catalog No.:BCC1893
CAS No.:1215493-56-3
- GR 144053 trihydrochloride
Catalog No.:BCC6998
CAS No.:1215333-48-4
- SR 58611A hydrochloride
Catalog No.:BCC7833
CAS No.:121524-09-2
- Salvianolic acid B; Lithospermic acid B; Danfensuan B
Catalog No.:BCC8249
CAS No.:121521-90-2
- PF-04991532
Catalog No.:BCC8094
CAS No.:1215197-37-7
- 5-Benzyloxyindole
Catalog No.:BCC8742
CAS No.:1215-59-4
- Daclatasvir (BMS-790052)
Catalog No.:BCC2533
CAS No.:1214735-16-6
- Ajugapantin A
Catalog No.:BCN3663
CAS No.:121449-67-0
- WZ4003
Catalog No.:BCC4363
CAS No.:1214265-58-3
- NBI 27914 hydrochloride
Catalog No.:BCC7124
CAS No.:1215766-76-9
- Gatifloxacin hydrochloride
Catalog No.:BCC4224
CAS No.:121577-32-0
- DMCM hydrochloride
Catalog No.:BCC7560
CAS No.:1215833-62-7
- Valspodar
Catalog No.:BCC2027
CAS No.:121584-18-7
- 6-Demethoxy-9'-deoxycleomiscosin A
Catalog No.:BCN7298
CAS No.:121587-18-6
- 6-Demethoxycleomiscosin A
Catalog No.:BCN7299
CAS No.:121587-20-0
- YM 298198 hydrochloride
Catalog No.:BCC7366
CAS No.:1216398-09-2
- SB 258585 hydrochloride
Catalog No.:BCC7216
CAS No.:1216468-02-8
- Kaempferol-3-O-(2',6'-di-O-trans-p-coumaroyl)-beta-D-glucopyranoside
Catalog No.:BCN1603
CAS No.:121651-61-4
- BX 513 hydrochloride
Catalog No.:BCC5940
CAS No.:1216540-18-9
- ZK 93423 hydrochloride
Catalog No.:BCC7227
CAS No.:1216574-52-5
- 2-Cyclopropyl-4-(4-fluorophenyl)-quinolyl-3-methanol
Catalog No.:BCC8574
CAS No.:121660-11-5
Design, synthesis, and pharmacological characterization of N- and O-substituted 5,6,7,8-tetrahydro-4H-isoxazolo[4,5-d]azepin-3-ol analogues: novel 5-HT(2A)/5-HT(2C) receptor agonists with pro-cognitive properties.[Pubmed:23301527]
J Med Chem. 2013 Feb 14;56(3):1211-27.
The isoxazol-3-one tautomer of the bicyclic isoxazole, 5,6,7,8-tetrahydro-4H-isoxazolo[4,5-d]azepin-3-ol (THAZ), has previously been shown to be a weak GABA(A) and glycine receptor antagonist. In the present study, the potential in this scaffold has been explored through the synthesis and pharmacological characterization of a series of N- and O-substituted THAZ analogues. The analogues N-Bn-THAZ (3d) and O-Bn-THAZ (4d) were found to be potent agonists of the human 5-HT(2A) and 5-HT(2C) receptors. Judging from an elaborate pharmacological profiling at numerous other CNS targets, the 3d analogue appears to be selective for the two receptors. Administration of 3d substantially improved the cognitive performance of mice in a place recognition Y-maze model, an effect fully reversible by coadministration of the selective 5-HT(2C) antagonist SB242084. In conclusion, as novel bioavailable cognitive enhancers that most likely mediate their effects through 5-HT(2A) and/or 5-HT(2C) receptors, the isoxazoles 3d and 4d constitute interesting leads for further medicinal chemistry development.
Genotoxicity of 2-(3-chlorobenzyloxy)-6-(piperazinyl)pyrazine, a novel 5-hydroxytryptamine2c receptor agonist for the treatment of obesity: role of metabolic activation.[Pubmed:17344339]
Drug Metab Dispos. 2007 Jun;35(6):848-58.
2-(3-Chlorobenzyloxy)-6-(piperazin-1-yl)pyrazine (3) is a potent and selective 5-HT(2C) agonist that exhibits dose-dependent inhibition of food intake and reduction in body weight in rats, making it an attractive candidate for treatment of obesity. However, examination of the genotoxicity potential of 3 in the Salmonella Ames assay using tester strains TA98, TA100, TA1535, and TA1537 revealed a metabolism (rat S9/NADPH)- and dose-dependent increase of reverse mutations in strains TA100 and TA1537. The increase in reverse mutations was attenuated upon coincubation with methoxylamine and glutathione. The irreversible and concentration-dependent incorporation of radioactivity in calf thymus DNA after incubations with [14C]3 in the presence of rat S9/NADPH suggested that 3 was bioactivated to a reactive intermediate that covalently bound DNA. In vitro metabolism studies on 3 with rat S9/NADPH in the presence of methoxylamine and cyanide led to the detection of amine and cyano conjugates of 3. The mass spectrum of the amine conjugate was consistent with condensation of amine with an aldehyde metabolite derived from hydroxylation of the secondary piperazine nitrogen-alpha-carbon bond. The mass spectrum of the cyano conjugate suggested a bioactivation pathway involving N-hydroxylation of the secondary piperazine nitrogen followed by two-electron oxidation to generate an electrophilic nitrone, which reacted with cyanide. The 3-chlorobenzyl motif in 3 was also bioactivated via initial aromatic ring hydroxylation followed by elimination to a quinone-methide species that reacted with glutathione or with the secondary piperazine ring nitrogen in 3 and its monohydroxylated metabolite(s). The metabolism studies described herein provide a mechanistic basis for the mutagenicity of 3.
CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity.[Pubmed:16949622]
Neuropharmacology. 2007 Feb;52(2):279-90.
CP-809,101 is a potent, functionally selective 5-HT(2C) agonist that displays approximately 100% efficacy in vitro. The aim of the present studies was to assess the efficacy of a selective 5-HT(2C) agonist in animal models predictive of antipsychotic-like efficacy and side-effect liability. Similar to currently available antipsychotic drugs, CP-809,101 dose-dependently inhibited conditioned avoidance responding (CAR, ED(50)=4.8 mg/kg, sc). The efficacy of CP-809,101 in CAR was completely antagonized by the concurrent administration of the 5-HT(2C) receptor antagonist, SB-224,282. CP-809,101 antagonized both PCP- and d-amphetamine-induced hyperactivity with ED(50) values of 2.4 and 2.9 mg/kg (sc), respectively and also reversed an apomorphine induced-deficit in prepulse inhibition. At doses up to 56 mg/kg, CP-809,101 did not produce catalepsy. Thus, the present results demonstrate that the 5-HT(2C) agonist, CP-809,101, has a pharmacological profile similar to that of the atypical antipsychotics with low extrapyramidal symptom liability. CP-809,101 was inactive in two animal models of antidepressant-like activity, the forced swim test and learned helplessness. However, CP-809,101 was active in novel object recognition, an animal model of cognitive function. These data suggest that 5-HT(2C) agonists may be a novel approach in the treatment of psychosis as well as for the improvement of cognitive dysfunction associated with schizophrenia.


