CFM 1571 hydrochloridesGC activator CAS# 1215548-30-3 |
- Biperiden HCl
Catalog No.:BCC4565
CAS No.:1235-82-1
- Darifenacin
Catalog No.:BCC1516
CAS No.:133099-04-4
- Darifenacin HBr
Catalog No.:BCC4567
CAS No.:133099-07-7
- Cevimeline hydrochloride hemihydrate
Catalog No.:BCC1471
CAS No.:153504-70-2
- Umeclidinium bromide
Catalog No.:BCC2022
CAS No.:869113-09-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1215548-30-3 | SDF | Download SDF |
| PubChem ID | 56972190 | Appearance | Powder |
| Formula | C23H29ClN4O3 | M.Wt | 444.95 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
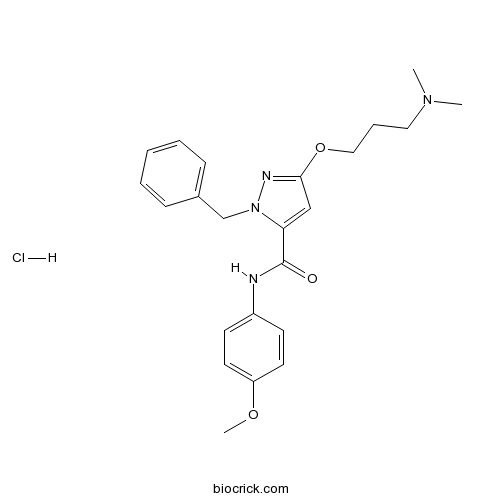
| Chemical Name | 2-benzyl-5-[3-(dimethylamino)propoxy]-N-(4-methoxyphenyl)pyrazole-3-carboxamide;hydrochloride | ||
| SMILES | CN(C)CCCOC1=NN(C(=C1)C(=O)NC2=CC=C(C=C2)OC)CC3=CC=CC=C3.Cl | ||
| Standard InChIKey | DFVHCFCSCUCAPD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H28N4O3.ClH/c1-26(2)14-7-15-30-22-16-21(27(25-22)17-18-8-5-4-6-9-18)23(28)24-19-10-12-20(29-3)13-11-19;/h4-6,8-13,16H,7,14-15,17H2,1-3H3,(H,24,28);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Soluble guanylyl cyclase (sGC) activator (EC50 = 5.49 μM). Does not activate adenylyl cyclase, shows no significant inhibition of phosphodiesterases and displays minimal inhibition of iNOS (25%) and nNOS (17%). Inhibits collagen-stimulated platelet aggregation in vitro (IC50 = 2.84 μM). |

CFM 1571 hydrochloride Dilution Calculator

CFM 1571 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2474 mL | 11.2372 mL | 22.4744 mL | 44.9489 mL | 56.1861 mL |
| 5 mM | 0.4495 mL | 2.2474 mL | 4.4949 mL | 8.9898 mL | 11.2372 mL |
| 10 mM | 0.2247 mL | 1.1237 mL | 2.2474 mL | 4.4949 mL | 5.6186 mL |
| 50 mM | 0.0449 mL | 0.2247 mL | 0.4495 mL | 0.899 mL | 1.1237 mL |
| 100 mM | 0.0225 mL | 0.1124 mL | 0.2247 mL | 0.4495 mL | 0.5619 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 2.84 μM for platelet
Soluble guanylate cyclase (sGC) is a key signal-transduction enzyme activated by nitric oxide (NO). Impaired bioavailability and/or responsiveness to endogenous NO has been implicated in the pathogenesis of cardiovascular and other diseases. CFM 1571 is a activator of the nitric oxide receptor, soluble guanylate cyclase.
In vitro: CFM 1571 demonstrated potent activation of soluble guanylate cyclase and potent inhibition of platelet aggregation. CFM 1571 also showed enzyme activity similar to benzydamine. CFM 1571 was additionally assayed against the NOS isoforms, and minimal inhibition was observed with iNOS (25%) and nNOS (17%). Furthermore CFM 1571 shows no significant activity at any of the other enzymes studied [1].
In vivo: Pharmacokinetic studies in rats showed that compound CFM 1571 exhibits modest oral bioavailability (12%). Furthermore 32 has an excellent selectivity profile notably showing no significant inhibition of phosphodiesterases or nitric oxide synthases [1].
Clinical trial: Up to now, CFM 1571 is still in the preclinical development stage.
Reference:
[1] Selwood DL, Brummell DG, Budworth J, Burtin GE, Campbell RO, Chana SS, Charles IG, Fernandez PA, Glen RC, Goggin MC, Hobbs AJ, Kling MR, Liu Q, Madge DJ, Meillerais S, Powell KL, Reynolds K, Spacey GD, Stables JN, Tatlock MA, Wheeler KA, Wishart G, Woo CK. Synthesis and biological evaluation of novel pyrazoles and indazoles as activators of the nitric oxide receptor, soluble guanylate cyclase. J Med Chem. 2001 Jan 4;44(1):78-93.
- RG2833
Catalog No.:BCC1893
CAS No.:1215493-56-3
- GR 144053 trihydrochloride
Catalog No.:BCC6998
CAS No.:1215333-48-4
- SR 58611A hydrochloride
Catalog No.:BCC7833
CAS No.:121524-09-2
- Salvianolic acid B; Lithospermic acid B; Danfensuan B
Catalog No.:BCC8249
CAS No.:121521-90-2
- PF-04991532
Catalog No.:BCC8094
CAS No.:1215197-37-7
- 5-Benzyloxyindole
Catalog No.:BCC8742
CAS No.:1215-59-4
- Daclatasvir (BMS-790052)
Catalog No.:BCC2533
CAS No.:1214735-16-6
- Ajugapantin A
Catalog No.:BCN3663
CAS No.:121449-67-0
- WZ4003
Catalog No.:BCC4363
CAS No.:1214265-58-3
- WZ8040
Catalog No.:BCC1075
CAS No.:1214265-57-2
- WZ3146
Catalog No.:BCC4004
CAS No.:1214265-56-1
- POM 1
Catalog No.:BCC7454
CAS No.:12141-67-2
- SB 242084
Catalog No.:BCC5949
CAS No.:1215566-78-1
- RS 100329 hydrochloride
Catalog No.:BCC5741
CAS No.:1215654-26-4
- CP-809101 hydrochloride
Catalog No.:BCC1499
CAS No.:1215721-40-6
- NBI 27914 hydrochloride
Catalog No.:BCC7124
CAS No.:1215766-76-9
- Gatifloxacin hydrochloride
Catalog No.:BCC4224
CAS No.:121577-32-0
- DMCM hydrochloride
Catalog No.:BCC7560
CAS No.:1215833-62-7
- Valspodar
Catalog No.:BCC2027
CAS No.:121584-18-7
- 6-Demethoxy-9'-deoxycleomiscosin A
Catalog No.:BCN7298
CAS No.:121587-18-6
- 6-Demethoxycleomiscosin A
Catalog No.:BCN7299
CAS No.:121587-20-0
- YM 298198 hydrochloride
Catalog No.:BCC7366
CAS No.:1216398-09-2
- SB 258585 hydrochloride
Catalog No.:BCC7216
CAS No.:1216468-02-8
- Kaempferol-3-O-(2',6'-di-O-trans-p-coumaroyl)-beta-D-glucopyranoside
Catalog No.:BCN1603
CAS No.:121651-61-4
NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential.[Pubmed:16955067]
Nat Rev Drug Discov. 2006 Sep;5(9):755-68.
Soluble guanylate cyclase (sGC) is a key signal-transduction enzyme activated by nitric oxide (NO). Impaired bioavailability and/or responsiveness to endogenous NO has been implicated in the pathogenesis of cardiovascular and other diseases. Current therapies that involve the use of organic nitrates and other NO donors have limitations, including non-specific interactions of NO with various biomolecules, lack of response and the development of tolerance following prolonged administration. Compounds that activate sGC in an NO-independent manner might therefore provide considerable therapeutic advantages. Here we review the discovery, biochemistry, pharmacology and clinical potential of haem-dependent sGC stimulators (including YC-1, BAY 41-2272, BAY 41-8543, CFM-1571 and A-350619) and haem-independent sGC activators (including BAY 58-2667 and HMR-1766).
Synthesis and biological evaluation of novel pyrazoles and indazoles as activators of the nitric oxide receptor, soluble guanylate cyclase.[Pubmed:11141091]
J Med Chem. 2001 Jan 4;44(1):78-93.
Database searching and compound screening identified 1-benzyl-3-(3-dimethylaminopropyloxy)indazole (benzydamine, 3) as a potent activator of the nitric oxide receptor, soluble guanylate cyclase. A comprehensive structure-activity relationship study surrounding 3 clearly showed that the indazole C-3 dimethylaminopropyloxy substituent was critical for enzyme activity. However replacement of the indazole ring of 3 by appropriately substituted pyrazoles maintained enzyme activity. Compounds were evaluated for inhibition of platelet aggregation and showed a general lipophilicity requirement. Aryl-substituted pyrazoles 32, 34, and 43 demonstrated potent activation of soluble guanylate cyclase and potent inhibition of platelet aggregation. Pharmacokinetic studies in rats showed that compound 32 exhibits modest oral bioavailability (12%). Furthermore 32 has an excellent selectivity profile notably showing no significant inhibition of phosphodiesterases or nitric oxide synthases.


