YM 298198 hydrochlorideHighly potent, selective non-competitive mGlu1 antagonist CAS# 1216398-09-2 |
- YM155
Catalog No.:BCC2251
CAS No.:781661-94-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1216398-09-2 | SDF | Download SDF |
| PubChem ID | 45073464 | Appearance | Powder |
| Formula | C18H23ClN4OS | M.Wt | 378.92 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
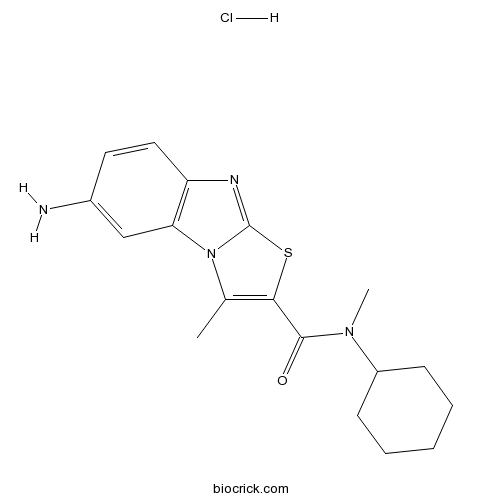
| Chemical Name | 7-amino-N-cyclohexyl-N,1-dimethyl-[1,3]thiazolo[3,2-a]benzimidazole-2-carboxamide;hydrochloride | ||
| SMILES | CC1=C(SC2=NC3=C(N12)C=C(C=C3)N)C(=O)N(C)C4CCCCC4.Cl | ||
| Standard InChIKey | WYTJVUVCSUWZTH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H22N4OS.ClH/c1-11-16(17(23)21(2)13-6-4-3-5-7-13)24-18-20-14-9-8-12(19)10-15(14)22(11)18;/h8-10,13H,3-7,19H2,1-2H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Non-competitive antagonist with high affinity and selectivity for mGlu1 receptors (Ki = 19 nM); inactive at other mGlu receptor subtypes (mGlu2-7), ionotropic receptors and glutamate transporters (IC50 > 10 μM). Inhibits glutamate-induced IP production more potently than CPCCOEt (IC50 values are 16 nM and 6.3 μM respectively), and is orally active in vivo, demonstrating an antinociceptive effect in hyperalgesic mice. Desmethyl derivative also available. |

YM 298198 hydrochloride Dilution Calculator

YM 298198 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6391 mL | 13.1954 mL | 26.3908 mL | 52.7816 mL | 65.977 mL |
| 5 mM | 0.5278 mL | 2.6391 mL | 5.2782 mL | 10.5563 mL | 13.1954 mL |
| 10 mM | 0.2639 mL | 1.3195 mL | 2.6391 mL | 5.2782 mL | 6.5977 mL |
| 50 mM | 0.0528 mL | 0.2639 mL | 0.5278 mL | 1.0556 mL | 1.3195 mL |
| 100 mM | 0.0264 mL | 0.132 mL | 0.2639 mL | 0.5278 mL | 0.6598 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-Demethoxycleomiscosin A
Catalog No.:BCN7299
CAS No.:121587-20-0
- 6-Demethoxy-9'-deoxycleomiscosin A
Catalog No.:BCN7298
CAS No.:121587-18-6
- Valspodar
Catalog No.:BCC2027
CAS No.:121584-18-7
- DMCM hydrochloride
Catalog No.:BCC7560
CAS No.:1215833-62-7
- Gatifloxacin hydrochloride
Catalog No.:BCC4224
CAS No.:121577-32-0
- NBI 27914 hydrochloride
Catalog No.:BCC7124
CAS No.:1215766-76-9
- CP-809101 hydrochloride
Catalog No.:BCC1499
CAS No.:1215721-40-6
- RS 100329 hydrochloride
Catalog No.:BCC5741
CAS No.:1215654-26-4
- SB 242084
Catalog No.:BCC5949
CAS No.:1215566-78-1
- CFM 1571 hydrochloride
Catalog No.:BCC5924
CAS No.:1215548-30-3
- RG2833
Catalog No.:BCC1893
CAS No.:1215493-56-3
- GR 144053 trihydrochloride
Catalog No.:BCC6998
CAS No.:1215333-48-4
- SB 258585 hydrochloride
Catalog No.:BCC7216
CAS No.:1216468-02-8
- Kaempferol-3-O-(2',6'-di-O-trans-p-coumaroyl)-beta-D-glucopyranoside
Catalog No.:BCN1603
CAS No.:121651-61-4
- BX 513 hydrochloride
Catalog No.:BCC5940
CAS No.:1216540-18-9
- ZK 93423 hydrochloride
Catalog No.:BCC7227
CAS No.:1216574-52-5
- 2-Cyclopropyl-4-(4-fluorophenyl)-quinolyl-3-methanol
Catalog No.:BCC8574
CAS No.:121660-11-5
- 2-Cyclopropyl-4-(4-fluorophenyl)quinoline-3-carboxaldehyde
Catalog No.:BCC8573
CAS No.:121660-37-5
- Trap 101
Catalog No.:BCC7390
CAS No.:1216621-00-9
- SCH 79797 dihydrochloride
Catalog No.:BCC7125
CAS No.:1216720-69-2
- BYK 191023 dihydrochloride
Catalog No.:BCC7506
CAS No.:1216722-25-6
- GSK 4112
Catalog No.:BCC7741
CAS No.:1216744-19-2
- ZK 93426 hydrochloride
Catalog No.:BCC7229
CAS No.:1216792-30-1
- CGP 20712 dihydrochloride
Catalog No.:BCC6893
CAS No.:1216905-73-5
The protective signaling of metabotropic glutamate receptor 1 Is mediated by sustained, beta-arrestin-1-dependent ERK phosphorylation.[Pubmed:20566651]
J Biol Chem. 2010 Aug 20;285(34):26041-8.
Metabotropic glutamate receptor 1 (mGlu1) is a G protein-coupled receptor that enhances the hydrolysis of membrane phosphoinositides. In addition to its role in synaptic transmission and plasticity, mGlu1 has been shown to be involved in neuroprotection and neurodegeneration. In this capacity, we have reported previously that in neuronal cells, mGlu1a exhibits the properties of a dependence receptor, inducing apoptosis in the absence of glutamate, while promoting neuronal survival in its presence (Pshenichkin, S., Dolinska, M., Klauzinska, M., Luchenko, V., Grajkowska, E., and Wroblewski, J. T. (2008) Neuropharmacology 55, 500-508). Here, using CHO cells expressing mGlu1a receptors, we show that the protective effect of glutamate does not rely on the classical mGlu1 signal transduction. Instead, mGlu1a protective signaling is mediated by a novel, G protein-independent, pathway which involves the activation of the MAPK pathway and a sustained phosphorylation of ERK, which is distinct from the G protein-mediated transient ERK phosphorylation. Moreover, the sustained phosphorylation of ERK and protective signaling through mGlu1a receptors require expression of beta-arrestin-1, suggesting a possible role for receptor internalization in this process. Our data reveal the existence of a novel, noncanonical signaling pathway associated with mGlu1a receptors, which mediates glutamate-induced protective signaling.
Radioligand binding properties and pharmacological characterization of 6-amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-a]benzimidazole-2-carboxamide (YM-298198), a high-affinity, selective, and noncompetitive antagonist of metabotropic glutamate receptor type 1.[Pubmed:15976016]
J Pharmacol Exp Ther. 2005 Oct;315(1):163-9.
Metabotropic glutamate receptor type 1 (mGluR1) is thought to play important roles in the neurotransmission and pathogenesis of several neurological disorders. Here, we describe the radioligand binding properties and pharmacological effects of a newly synthesized, high-affinity, selective, and noncompetitive mGluR1 antagonist, 6-amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-a]benzimidazole-2-carboxamide (YM-298198). YM-298198 inhibited glutamate-induced inositol phosphate production in mGluR1-NIH3T3 cells with an IC50 of 16 +/- 5.8 nM in a noncompetitive manner. Its radiolabeled form, [3H]YM-298198, bound to mGluR1-NIH3T3 cell membranes with a KD of 32 +/- 8.5 nM and a Bmax of 2297 +/- 291 fmol/mg protein. In ligand displacement experiments using rat cerebellum membrane, an existing noncompetitive mGluR1 antagonist 7-(hydroxyimino)cyclo-propa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt) competitively displaced [3H]YM-298198 binding, although glutamate and other mGluR1 ligands acting on a glutamate site failed to inhibit [3H]YM-298198 binding, suggesting that YM-298198 binds to CPCCOEt (allosteric) binding sites but not to glutamate (agonist) binding sites. Specificity was demonstrated for mGluR1 over mGluR subtypes 2 to 7, ionotropic glutamate receptors, and other receptor, transporter, and ion channel targets. In in vivo experiments, orally administered YM-298198 showed a significant analgesic effect in streptozotocin-induced hyperalgesic mice at doses (30 mg/kg) that did not cause Rotarod performance impairment, indicating that it is also useful even for in vivo experiments. In conclusion, YM-298198 is a newly synthesized, high-affinity, selective, and noncompetitive antagonist of mGluR1 that will be a useful pharmacological tool due to its highly active properties in vitro and in vivo. Its radiolabeled form [3H]YM-298198 will also be a valuable tool for future investigation of the mGluR1.


