Cevimeline hydrochloride hemihydrateAgonist of muscarinic receptor (M1/M3) CAS# 153504-70-2 |
- Cevimeline
Catalog No.:BCC1470
CAS No.:107233-08-9
- Biperiden HCl
Catalog No.:BCC4565
CAS No.:1235-82-1
- Darifenacin
Catalog No.:BCC1516
CAS No.:133099-04-4
- Darifenacin HBr
Catalog No.:BCC4567
CAS No.:133099-07-7
- Umeclidinium bromide
Catalog No.:BCC2022
CAS No.:869113-09-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 153504-70-2 | SDF | Download SDF |
| PubChem ID | 66577068 | Appearance | Powder |
| Formula | C10H20ClNO2S | M.Wt | 253.79 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 50 mg/mL (204.27 mM) *"≥" means soluble, but saturation unknown. | ||
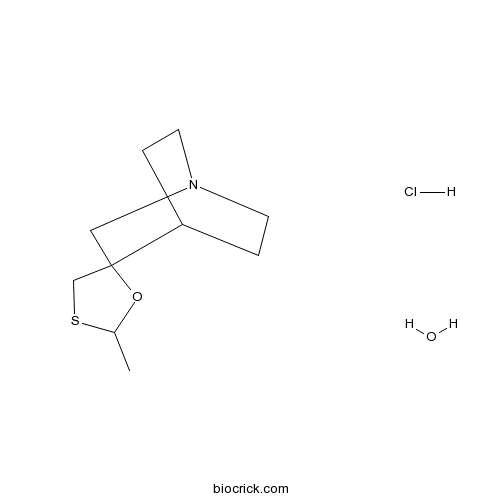
| Chemical Name | 2-methylspiro[1,3-oxathiolane-5,3'-1-azabicyclo[2.2.2]octane];hydrate;hydrochloride | ||
| SMILES | CC1OC2(CN3CCC2CC3)CS1.O.Cl | ||
| Standard InChIKey | JKJRNPOQQPBPOV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H17NOS.ClH.H2O/c1-8-12-10(7-13-8)6-11-4-2-9(10)3-5-11;;/h8-9H,2-7H2,1H3;1H;1H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cevimeline hydrochloride hemihydrate, a novel muscarinic receptor agonist, is a candidate therapeutic drug for xerostomia in Sjogren's syndrome.
IC50 value:
Target: mAChR
The general pharmacol. properties of this drug on the gastrointestinal, urinary, and reproductive systems and other tissues were investigated in mice, rats, guinea pigs, rabbits, and dogs. The in vitro metab. of SNI-2011 was also evaluated with rat and dog liver microsomes. After oral administration, plasma concns. of SNI-2011 reached to Cmax within 1 h in both species, suggesting that SNI-2011 was quickly absorbed, and then decreased with a t1/2 of 0.4-1.1 h. The bioavailability was 50% and 30% in rats and dogs, resp. Major metabolites in plasma were both S- and N-oxidized metabolites in rats and only N-oxidized metabolite in dogs, indicating that a large species difference was obsd. in the metab. of SNI-2011. Sex difference was also obsd. in the pharmacokinetics of SNI-2011 in rats, but not in dogs. In the in vitro study, chem. inhibition and pH-dependent studies revealed that the sulfoxidn. and N-oxidn. of SNI-2011 were mediated by cytochrome P 450 (CYP) and flavin-contg. monooxygenase (FMO), resp., in both species. In addn., CYP2D and CYP3A were mainly responsible for the sulfoxidn. in rat liver microsomes. References: | |||||

Cevimeline hydrochloride hemihydrate Dilution Calculator

Cevimeline hydrochloride hemihydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9403 mL | 19.7013 mL | 39.4027 mL | 78.8053 mL | 98.5066 mL |
| 5 mM | 0.7881 mL | 3.9403 mL | 7.8805 mL | 15.7611 mL | 19.7013 mL |
| 10 mM | 0.394 mL | 1.9701 mL | 3.9403 mL | 7.8805 mL | 9.8507 mL |
| 50 mM | 0.0788 mL | 0.394 mL | 0.7881 mL | 1.5761 mL | 1.9701 mL |
| 100 mM | 0.0394 mL | 0.197 mL | 0.394 mL | 0.7881 mL | 0.9851 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cevimeline hydrochloride hemihydrate is a novel and selective agonist of muscarinic acetylcholine receptor [1].
Muscarinic acetylcholine receptors (mAChRs) are acetylcholine receptors and mediate serous-saliva secretion and tear secretion. It is more sensitive to muscarine than nicotine [1].
Cevimeline hydrochloride hemihydrate induced contractions of isolated guinea pig ilea and trachea with EC50 values of 3.5 and 3 μM, respectively. Binding studies indicated that Cevimeline hydrochloride hemihydrate was a potent and highly selective M1-type muscarinic agonist. Also, it had a higher affinity for M1 receptors than other M1 agonists [2].
In normal rats and mice, X-irradiated saliva secretion defective rats and two strains of autoimmune disease mice, intraduodenal administrations of SNI-2011 (3-30 mg/kg) increased saliva and tear secretions in a dose-dependent way, which suggested that Cevimeline hydrochloride hemihydrate directly stimulated muscarinic receptors in salivary and lacrimal glands for saliva and tear secretions [1].
References:
[1]. Iga Y, Arisawa H, Ogane N, et al. (+/-)-cis-2-methylspiro[1,3-oxathiolane-5,3'-quinuclidine] hydrochloride, hemihydrate (SNI-2011, cevimeline hydrochloride) induces saliva and tear secretions in rats and mice: the role of muscarinic acetylcholine receptors. Jpn J Pharmacol, 1998, 78(3): 373-380.
[2]. Fisher A, Brandeis R, Karton I, et al. (+-)-cis-2-methyl-spiro(1,3-oxathiolane-5,3') quinuclidine, an M1 selective cholinergic agonist, attenuates cognitive dysfunctions in an animal model of Alzheimer's disease. J Pharmacol Exp Ther, 1991, 257(1): 392-403.
- Xanthinin
Catalog No.:BCN1686
CAS No.:153483-31-9
- Carbazeran citrate
Catalog No.:BCC6173
CAS No.:153473-94-0
- Desmethoxy yangonin
Catalog No.:BCN2295
CAS No.:15345-89-8
- Fexofenadine HCl
Catalog No.:BCC4542
CAS No.:153439-40-8
- WHI-P180 hydrochloride
Catalog No.:BCC4243
CAS No.:153437-55-9
- PD153035 hydrochloride
Catalog No.:BCC3617
CAS No.:153436-54-5
- AG-1478
Catalog No.:BCC3717
CAS No.:153436-53-4
- Gavestinel
Catalog No.:BCC7340
CAS No.:153436-38-5
- Taxcultine
Catalog No.:BCN6948
CAS No.:153415-46-4
- Taxol C
Catalog No.:BCN6941
CAS No.:153415-45-3
- 7-Epi-docetaxel
Catalog No.:BCC5411
CAS No.:153381-68-1
- Ginkgolide K
Catalog No.:BCN8209
CAS No.:153355-70-5
- [D-Trp34]-Neuropeptide Y
Catalog No.:BCC7690
CAS No.:153549-84-9
- Bexarotene
Catalog No.:BCC3737
CAS No.:153559-49-0
- D-Menthol
Catalog No.:BCN4973
CAS No.:15356-60-2
- DL-Menthol
Catalog No.:BCN5950
CAS No.:15356-70-4
- 13-Hydroxylupanine
Catalog No.:BCN3204
CAS No.:15358-48-2
- NS 1619
Catalog No.:BCC7779
CAS No.:153587-01-0
- GNE-9605
Catalog No.:BCC5458
CAS No.:1536200-31-3
- Dofequidar fumarate
Catalog No.:BCC4177
CAS No.:153653-30-6
- H-Ile-OEt.HCl
Catalog No.:BCC2961
CAS No.:15366-32-3
- 9,10-Bis(3,5-dihydroxyphenyl)anthracene
Catalog No.:BCC8793
CAS No.:153715-08-3
- Eriodictyol-8-glucoside
Catalog No.:BCN8029
CAS No.:153733-96-1
- Dioxopromethazine hydrochloride
Catalog No.:BCC8946
CAS No.:15374-15-9
(+/-)-cis-2-methylspiro[1,3-oxathiolane-5,3'-quinuclidine] hydrochloride, hemihydrate (SNI-2011, cevimeline hydrochloride) induces saliva and tear secretions in rats and mice: the role of muscarinic acetylcholine receptors.[Pubmed:9869272]
Jpn J Pharmacol. 1998 Nov;78(3):373-80.
We investigated effects of (+/-)-cis-2-methylspiro[1,3-oxathiolane-5,3'-quinuclidine] hydrochloride, hemihydrate (SNI-2011, cevimeline hydrochloride), a rigid analogue of acetylcholine, on saliva and tear secretions in rats and mice to evaluate its therapeutical efficacy for xerostomia and xerophthalmia in patients with Sjogren's syndrome and X-ray exposure in the head and neck. Intraduodenal administrations of SNI-2011 increased saliva secretion in a dose-dependent manner at doses ranging from 3 to 30 mg/kg in normal rats and mice, two strains of autoimmune disease mice and X-irradiated saliva secretion defective rats. The salivation elicited by SNI-2011 was completely inhibited by atropine. A similar atropine-sensitive response was observed in tear secretion. In rat submandibular/sublingual gland membranes, [3H]quinuclidinyl benzilate (QNB) binding was saturable, and Scatchard plot analysis revealed a single population of binding sites with a Kd of 22 pM and a maximal binding capacity of 60 fmol/mg protein. The competitive inhibition curve of the [3H]QNB binding by SNI-2011 was obtained, and its dissociation constant value calculated from IC50 was 1-2 microM. These results suggest that SNI-2011 increases saliva and tear secretions through a direct stimulation to muscarinic receptors in salivary and lacrimal glands, and they suggest that SNI-2011 should be beneficial to patients with Sjogren's syndrome and X-ray exposure in the head and neck.


