XanthininCAS# 153483-31-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 153483-31-9 | SDF | Download SDF |
| PubChem ID | 160533 | Appearance | Powder |
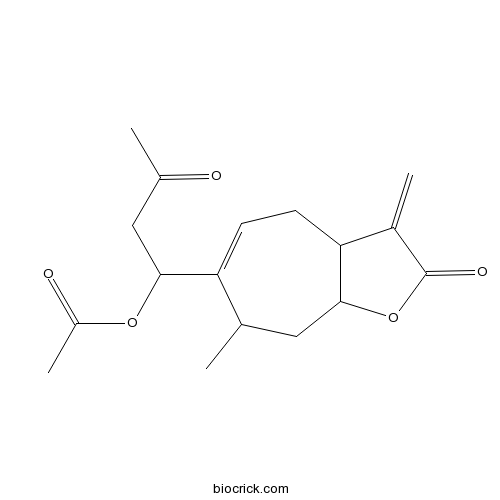
| Formula | C17H22O5 | M.Wt | 306.4 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [1-(7-methyl-3-methylidene-2-oxo-4,7,8,8a-tetrahydro-3aH-cyclohepta[b]furan-6-yl)-3-oxobutyl] acetate | ||
| SMILES | CC1CC2C(CC=C1C(CC(=O)C)OC(=O)C)C(=C)C(=O)O2 | ||
| Standard InChIKey | DPSCQKGSAHTWSP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H22O5/c1-9-7-15-14(11(3)17(20)22-15)6-5-13(9)16(8-10(2)18)21-12(4)19/h5,9,14-16H,3,6-8H2,1-2,4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Xanthinin is a plant growth-regulating compound from Xanthium pennsylvanicum. 2. Xanthinin is a phytotoxic xanthanolide, can inhibit the growth of plants. |

Xanthinin Dilution Calculator

Xanthinin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2637 mL | 16.3185 mL | 32.6371 mL | 65.2742 mL | 81.5927 mL |
| 5 mM | 0.6527 mL | 3.2637 mL | 6.5274 mL | 13.0548 mL | 16.3185 mL |
| 10 mM | 0.3264 mL | 1.6319 mL | 3.2637 mL | 6.5274 mL | 8.1593 mL |
| 50 mM | 0.0653 mL | 0.3264 mL | 0.6527 mL | 1.3055 mL | 1.6319 mL |
| 100 mM | 0.0326 mL | 0.1632 mL | 0.3264 mL | 0.6527 mL | 0.8159 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Carbazeran citrate
Catalog No.:BCC6173
CAS No.:153473-94-0
- Desmethoxy yangonin
Catalog No.:BCN2295
CAS No.:15345-89-8
- Fexofenadine HCl
Catalog No.:BCC4542
CAS No.:153439-40-8
- WHI-P180 hydrochloride
Catalog No.:BCC4243
CAS No.:153437-55-9
- PD153035 hydrochloride
Catalog No.:BCC3617
CAS No.:153436-54-5
- AG-1478
Catalog No.:BCC3717
CAS No.:153436-53-4
- Gavestinel
Catalog No.:BCC7340
CAS No.:153436-38-5
- Taxcultine
Catalog No.:BCN6948
CAS No.:153415-46-4
- Taxol C
Catalog No.:BCN6941
CAS No.:153415-45-3
- 7-Epi-docetaxel
Catalog No.:BCC5411
CAS No.:153381-68-1
- Ginkgolide K
Catalog No.:BCN8209
CAS No.:153355-70-5
- SCR7
Catalog No.:BCC3978
CAS No.:1533426-72-0
- Cevimeline hydrochloride hemihydrate
Catalog No.:BCC1471
CAS No.:153504-70-2
- [D-Trp34]-Neuropeptide Y
Catalog No.:BCC7690
CAS No.:153549-84-9
- Bexarotene
Catalog No.:BCC3737
CAS No.:153559-49-0
- D-Menthol
Catalog No.:BCN4973
CAS No.:15356-60-2
- DL-Menthol
Catalog No.:BCN5950
CAS No.:15356-70-4
- 13-Hydroxylupanine
Catalog No.:BCN3204
CAS No.:15358-48-2
- NS 1619
Catalog No.:BCC7779
CAS No.:153587-01-0
- GNE-9605
Catalog No.:BCC5458
CAS No.:1536200-31-3
- Dofequidar fumarate
Catalog No.:BCC4177
CAS No.:153653-30-6
- H-Ile-OEt.HCl
Catalog No.:BCC2961
CAS No.:15366-32-3
- 9,10-Bis(3,5-dihydroxyphenyl)anthracene
Catalog No.:BCC8793
CAS No.:153715-08-3
- Eriodictyol-8-glucoside
Catalog No.:BCN8029
CAS No.:153733-96-1
Optimization of xanthatin extraction from Xanthium spinosum L. and its cytotoxic, anti-angiogenesis and antiviral properties.[Pubmed:25481815]
Eur J Med Chem. 2015 Jan 27;90:491-6.
The aqueous extraction of the sesquiterpene lactone xanthatin from Xanthium spinosum L. favours the conversion of Xanthinin (1) to xanthatin (2) via the loss of acetic acid. The cytotoxic (Hep-G2 and L1210 human cell lines) and antiviral activities of isolated xanthatin are established. This natural compound shows significant cytotoxicity against the Hep-G2 cell line and our experimental results reveal its strong anti-angiogenesis capacity in vitro. The structure of xanthatin is determined by spectroscopic methods and for the first time confirmed by X-ray diffraction.
Phytochemical compositions and biological activities of essential oil from Xanthium strumarium L.[Pubmed:25898416]
Molecules. 2015 Apr 17;20(4):7034-47.
The chemical composition of the essential oil (EO) from fresh cocklebur (Xanthium strumarium L.) leaves was investigated by GC-MS. The antimicrobial activity of the EO was tested against Gram-positive and Gram-negative bacteria and fungi. Scolicidal activity was assayed against Echinococcus granulosus protoscolices. In total, 34 compounds were identified, accounting for 98.96% of the EO. The main compounds in the EO were cis-beta-guaiene (34.2%), limonene (20.3%), borneol (11.6%), bornyl acetate (4.5%), beta-cubebene (3.8%), sabinene (3.6%), phytol (3.1%), beta-selinene (2.8%), camphene (2.2%), alpha-cubebene (2.4%), beta-caryophyllene (1.9%), alpha-pinene (1.8%) and Xanthinin (1.04%). The antibacterial and antifungal screening of the EO showed that all assayed concentrations significantly inhibited the growth of Staphylococcus aureus, Bacillus subtilis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Candida albicans and Aspergillus niger (MIC = 0.5 +/- 0.1, 1.3 +/- 0.0, 4.8 +/- 0.0, 20.5 +/- 0.3, 55.2 +/- 0.0 and 34.3 +/- 0.0 microg/mL, respectively). The scolicidal assay indicated that the EO exhibited a significant activity against E. granulosus protoscolices. To the best of our knowledge, this is the first report on the scolicidal activity of X. strumarium. Because of the emergence of antimicrobial drug resistance, the study of new effective natural chemotherapeutic agents, such as the X. strumarium EO, possibly with low side effects, represents a very promising approach in biomedical research.


