PD153035 hydrochloridePotent EGFR inhibitor CAS# 153436-54-5 |
- SKLB610
Catalog No.:BCC3647
CAS No.:1125780-41-7
- CO-1686 (AVL-301)
Catalog No.:BCC1490
CAS No.:1374640-70-6
- Gefitinib hydrochloride
Catalog No.:BCC1591
CAS No.:184475-55-6
- Pelitinib (EKB-569)
Catalog No.:BCC1118
CAS No.:257933-82-7
- SU 9516
Catalog No.:BCC2398
CAS No.:377090-84-1
- Lapatinib Ditosylate
Catalog No.:BCC2083
CAS No.:388082-78-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 153436-54-5 | SDF | Download SDF |
| PubChem ID | 4705 | Appearance | Powder |
| Formula | C16H14BrN3O2 | M.Wt | 360.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ZM 252868; Tyrphostin AG 1517; AG 1517; SU 5271 | ||
| Solubility | DMSO : 33.33 mg/mL (92.53 mM; Need ultrasonic) | ||
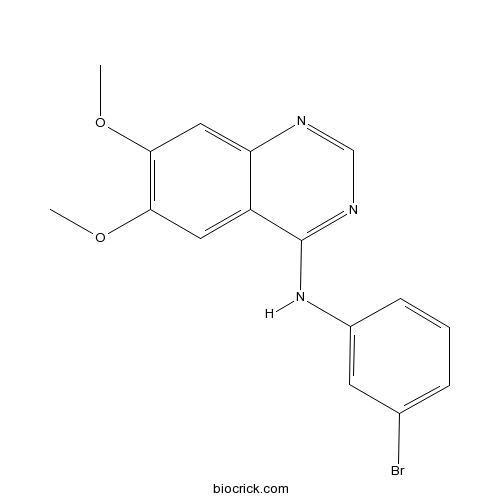
| Chemical Name | N-(3-bromophenyl)-6,7-dimethoxyquinazolin-4-amine | ||
| SMILES | COC1=C(C=C2C(=C1)C(=NC=N2)NC3=CC(=CC=C3)Br)OC | ||
| Standard InChIKey | LSPANGZZENHZNJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H14BrN3O2/c1-21-14-7-12-13(8-15(14)22-2)18-9-19-16(12)20-11-5-3-4-10(17)6-11/h3-9H,1-2H3,(H,18,19,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | PD153035 is a potent and specific inhibitor of EGFR with Ki and IC50 of 5.2 pM and 29 pM. | |||||
| Targets | EGFR | EGFR | ||||
| IC50 | 5.2 pM (Ki) | 29 pM | ||||

PD153035 hydrochloride Dilution Calculator

PD153035 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7762 mL | 13.8812 mL | 27.7624 mL | 55.5247 mL | 69.4059 mL |
| 5 mM | 0.5552 mL | 2.7762 mL | 5.5525 mL | 11.1049 mL | 13.8812 mL |
| 10 mM | 0.2776 mL | 1.3881 mL | 2.7762 mL | 5.5525 mL | 6.9406 mL |
| 50 mM | 0.0555 mL | 0.2776 mL | 0.5552 mL | 1.1105 mL | 1.3881 mL |
| 100 mM | 0.0278 mL | 0.1388 mL | 0.2776 mL | 0.5552 mL | 0.6941 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PD153035 (4-(3-Bromoanilino)-6,7-dimethoxyquinazoline) is an extremely potent epidermal growth factor receptor (EGFR) inhibitor that competitively binds at the ATP site with the half maximal inhibition concentration IC50 of 0.025 nM resulting in the inhibition of the tyrosine kinase activity of the EGFR [1].
PD153035 has been found to inhibit EGF-dependent EGFR phosphorylation in a variety of human cancer cell lines over-expressing EGFRs, which include A431, Difi, DU145, MDA-MB-468, ME180 and C4i, with IC50 of 0.22 μM, 0.3 μM, 0.4 μM, 0.68 μM, 0.95 μM and 2.5 μM respectively leading to suppression of proliferation and clonogenicity in those cell lines [2].
Besides exerting potent inhibition against EGFRs, PD153035 also inhibits the closely related HER2/neu receptor but to a lesser degree [2].
References:
[1] Bridges AJ, Zhou H, Cody DR, Rewcastle GW, McMichael A, Showalter HD, Fry DW, Kraker AJ, Denny WA. Tyrosine kinase inhibitors. 8. An unusually steep structure-activity relationship for analogues of 4-(3-bromoanilino)-6,7-dimethoxyquinazoline (PD 153035), a potent inhibitor of the epidermal growth factor receptor. J Med Chem. 1996 Jan 5;39(1):267-76.
[2] Bos M, Mendelsohn J, Kim YM, Albanell J, Fry DW, Baselga J. PD153035, a tyrosine kinase inhibitor, prevents epidermal growth factor receptor activation and inhibits growth of cancer cells in a receptor number-dependent manner. Clin Cancer Res. 1997 Nov;3(11):2099-106.
- AG-1478
Catalog No.:BCC3717
CAS No.:153436-53-4
- Gavestinel
Catalog No.:BCC7340
CAS No.:153436-38-5
- Taxcultine
Catalog No.:BCN6948
CAS No.:153415-46-4
- Taxol C
Catalog No.:BCN6941
CAS No.:153415-45-3
- 7-Epi-docetaxel
Catalog No.:BCC5411
CAS No.:153381-68-1
- Ginkgolide K
Catalog No.:BCN8209
CAS No.:153355-70-5
- SCR7
Catalog No.:BCC3978
CAS No.:1533426-72-0
- DFB
Catalog No.:BCC7130
CAS No.:15332-10-2
- 4,4'-Bis(2-benzoxazolyl)stilbene
Catalog No.:BCC8656
CAS No.:1533-45-5
- ML355
Catalog No.:BCC8060
CAS No.:1532593-30-8
- Cilomilast
Catalog No.:BCC2283
CAS No.:153259-65-5
- Taxayunnansin A
Catalog No.:BCN1685
CAS No.:153229-31-3
- WHI-P180 hydrochloride
Catalog No.:BCC4243
CAS No.:153437-55-9
- Fexofenadine HCl
Catalog No.:BCC4542
CAS No.:153439-40-8
- Desmethoxy yangonin
Catalog No.:BCN2295
CAS No.:15345-89-8
- Carbazeran citrate
Catalog No.:BCC6173
CAS No.:153473-94-0
- Xanthinin
Catalog No.:BCN1686
CAS No.:153483-31-9
- Cevimeline hydrochloride hemihydrate
Catalog No.:BCC1471
CAS No.:153504-70-2
- [D-Trp34]-Neuropeptide Y
Catalog No.:BCC7690
CAS No.:153549-84-9
- Bexarotene
Catalog No.:BCC3737
CAS No.:153559-49-0
- D-Menthol
Catalog No.:BCN4973
CAS No.:15356-60-2
- DL-Menthol
Catalog No.:BCN5950
CAS No.:15356-70-4
- 13-Hydroxylupanine
Catalog No.:BCN3204
CAS No.:15358-48-2
- NS 1619
Catalog No.:BCC7779
CAS No.:153587-01-0
A novel hybrid peptide targeting EGFR-expressing cancers.[Pubmed:21112771]
Eur J Cancer. 2011 Mar;47(5):773-83.
Several potential molecular-targeted anticancer drugs focus on the inhibition of receptor tyrosine kinase and tumour growth, but these tyrosine kinase inhibitors (TKI) have been reported that the mutations of kinase-related signal molecule genes in cancer cells lead to the drug resistance. To overcome this issue, we have designed a novel targeting anticancer 'hybrid-peptide' EGFR-lytic peptide, in which epidermal growth factor receptor (EGFR) binding peptide is conjugated with a newly designed lytic-type peptide containing cationic-rich amino acids that disintegrates the cell membrane to kill cancer cells. In this report, cytotoxic activity of EGFR-lytic peptide was investigated in various human cancer and normal cell lines. It was found that the resulting conformational change in the novel lytic peptide enabled it to bind selectively to the membrane of cancer cells, and due to its acquired synergistic action, hybrid peptide demonstrated selective destruction of cancer cells as swiftly as 10 min after exposure. Treatment with EGFR-lytic peptide exerted a sufficient in vitro cytotoxic activity against TKI-resistant cancer cells with K-ras mutations. Moreover, in vivo analyses revealed that this peptide displayed significant antitumour activity in mouse xenograft models of both human K-ras mutation negative and positive cancers. Thus, hybrid peptide can be a unique and powerful tool for a new cancer-targeted therapy.
Gefitinib, but not erlotinib, is a possible inducer of Fra-1-mediated interstitial lung disease.[Pubmed:23324306]
Keio J Med. 2012;61(4):120-7.
Gefitinib is an anticancer drug developed to inhibit the tyrosine kinase activity of the epidermal growth factor receptor (EGFR). Two structurally-related EGFR tyrosine kinase inhibitors, gefitinib (Iressa) and erlotinib (Tarceva), are used as oral chemotherapy by patients with non-small-cell lung cancer. Immediately after introduction of gefitinib to clinical practice, interstitial lung disease was identified as a life-threatening adverse effect, although this condition can be well managed. It is still unclear whether gefitinib and other EGFR inhibitors induce similar adverse effects in lung. We previously established mouse models of interstitial lung disease in which gefitinib induces expression of Fosl1 (which encodes the AP-1 transcription factor Fra-1) in the presence of exogenous or endogenous Toll-like receptor ligands, leading to abnormal cytokine and chemokine expression. Here, we compared and monitored the effects of EGFR inhibitors gefitinib, erlotinib and AG1517 (PD153035) on the mRNA expression levels of Fosl1, Tnf and Ccl2. Unexpectedly, gefitinib, but not the other tyrosine kinase inhibitors, elicited the Fosl1 expression profile proposed to be predictive of interstitial lung disease, suggesting that gefitinib-induced interstitial lung disease is an off-target effect not elicited by erlotinib.
Inhibition of epidermal growth factor receptor tyrosine kinase ameliorates collagen-induced arthritis.[Pubmed:22393153]
J Immunol. 2012 Apr 1;188(7):3513-21.
Rheumatoid arthritis (RA) is an autoimmune synovitis characterized by the formation of pannus and the destruction of cartilage and bone in the synovial joints. Although immune cells, which infiltrate the pannus and promote inflammation, play a prominent role in the pathogenesis of RA, other cell types also contribute. Proliferation of synovial fibroblasts, for example, underlies the formation of the pannus, while proliferation of endothelial cells results in neovascularization, which supports the growth of the pannus by supplying it with nutrients and oxygen. The synovial fibroblasts also promote inflammation in the synovium by producing cytokines and chemokines. Finally, osteoclasts cause the destruction of bone. In this study, we show that erlotinib, an inhibitor of the tyrosine kinase epidermal growth factor receptor (EGFR), reduces the severity of established collagen-induced arthritis, a mouse model of RA, and that it does so by targeting synovial fibroblasts, endothelial cells, and osteoclasts. Erlotinib-induced attenuation of autoimmune arthritis was associated with a reduction in number of osteoclasts and blood vessels, and erlotinib inhibited the formation of murine osteoclasts and the proliferation of human endothelial cells in vitro. Erlotinib also inhibited the proliferation and cytokine production of human synovial fibroblasts in vitro. Moreover, EGFR was highly expressed and activated in the synovium of mice with collagen-induced arthritis and patients with RA. Taken together, these findings suggest that EGFR plays a central role in the pathogenesis of RA and that EGFR inhibition may provide benefits in the treatment of RA.
Tyrosine kinase inhibitors influence ABCG2 expression in EGFR-positive MDCK BCRP cells via the PI3K/Akt signaling pathway.[Pubmed:22354538]
ChemMedChem. 2012 Apr;7(4):650-62.
Multidrug resistance observed in cancer chemotherapy is commonly attributed to overexpression of efflux transporter proteins. These proteins act as ATP-dependent drug efflux pumps, actively extruding chemotherapeutic agents from cells and causing a decrease in intracellular drug accumulation. Besides the well-recognized role of P-glycoprotein (P-gp, ABCB1), the breast cancer resistance protein (BCRP, ABCG2) is becoming increasingly accepted as playing an important role in multidrug resistance. In contrast to P-glycoprotein, only a few inhibitors of ABCG2 are known. According to the literature, tyrosine kinase inhibitors (TKIs) can be considered to be broad-spectrum inhibitors, interacting with ABCB1, ABCC1 and ABCG2. Here, we investigated seven different TKIs, gefitinib, erlotinib, AG1478, PD158780, PD153035, nilotinib and imatinib, for their potential to restore ABCG2 sensitivity to cells. Furthermore, we analyzed the alteration of ABCG2 expression caused by TKIs and demonstrated that EGFR inhibitors such as gefitinib and PD158780 reduced both total and surface expression of ABCG2 in EGRF-positive MDCK BCRP cells by interaction with the PI3K/Akt signaling pathway. The reduced ABCG2 content led to an increased effect of XR9577, a well-known ABCG2 modulator, lowering the concentration required for half maximal inhibition. On the other hand, BCR-ABL inhibitors had no influence on ABCG2 expression and modulator activity. Interestingly, a combination of an EGFR inhibitor with the PI3K/Akt inhibitor LY294002 led to a significant reduction of ABCG2 expression at low concentrations of the drugs. Based on our results, we assume that EGFR exerts a post-transcriptional enhancing effect on ABCG2 expression via the PI3K/Akt signaling pathway, which can be attenuated by EGFR inhibitors. Blocking the key signaling pathway regulating ABCG2 expression with EGFR inhibitors, combined with the inhibition of ABCG2 with potent modulators might be a promising approach to circumvent MDR in cancer cells.


