CevimelineMuscarinic receptor agonist CAS# 107233-08-9 |
- Biperiden HCl
Catalog No.:BCC4565
CAS No.:1235-82-1
- Darifenacin
Catalog No.:BCC1516
CAS No.:133099-04-4
- Darifenacin HBr
Catalog No.:BCC4567
CAS No.:133099-07-7
- Cevimeline hydrochloride hemihydrate
Catalog No.:BCC1471
CAS No.:153504-70-2
- Umeclidinium bromide
Catalog No.:BCC2022
CAS No.:869113-09-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 107233-08-9 | SDF | Download SDF |
| PubChem ID | 83898 | Appearance | Powder |
| Formula | C10H17NOS | M.Wt | 199.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (250.87 mM; Need ultrasonic) | ||
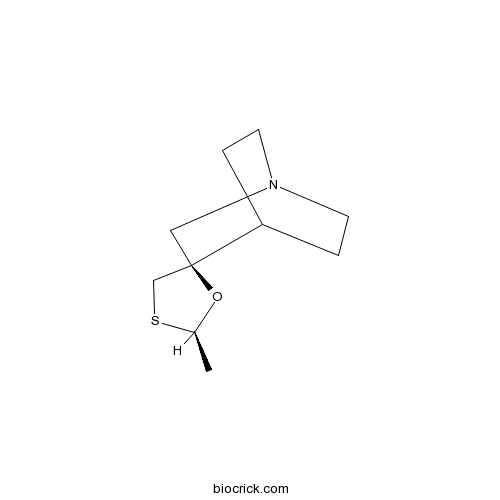
| Chemical Name | (2R,5R)-2-methylspiro[1,3-oxathiolane-5,3'-1-azabicyclo[2.2.2]octane] | ||
| SMILES | CC1OC2(CN3CCC2CC3)CS1 | ||
| Standard InChIKey | WUTYZMFRCNBCHQ-PSASIEDQSA-N | ||
| Standard InChI | InChI=1S/C10H17NOS/c1-8-12-10(7-13-8)6-11-4-2-9(10)3-5-11/h8-9H,2-7H2,1H3/t8-,10-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cevimeline (Evoxac) is a parasympathomimetic and muscarinic agonist, with particular effect on M3 receptors; used in the treatment of dry mouth associated with sjogren's syndrome.
IC50 value:
Target: M3 receptor References: | |||||

Cevimeline Dilution Calculator

Cevimeline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0171 mL | 25.0853 mL | 50.1706 mL | 100.3412 mL | 125.4264 mL |
| 5 mM | 1.0034 mL | 5.0171 mL | 10.0341 mL | 20.0682 mL | 25.0853 mL |
| 10 mM | 0.5017 mL | 2.5085 mL | 5.0171 mL | 10.0341 mL | 12.5426 mL |
| 50 mM | 0.1003 mL | 0.5017 mL | 1.0034 mL | 2.0068 mL | 2.5085 mL |
| 100 mM | 0.0502 mL | 0.2509 mL | 0.5017 mL | 1.0034 mL | 1.2543 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cevimeline is a muscarinic receptor agonist especially on the M1 and M3 receptors. [1]
Cevimeline has been approved for use against symptoms of dry mouth by activating the M3 receptors of the parasympathetic nervous system. Cevimeline is effective and safe in improving symptoms of dry eye with 20 mg three times per day [2]. Cevimeline increased the intracellular Ca+ level in parotid gland acinar cells over 1 μM and rat, enhanced the excitability via muscarinic receptors, thereby, cevimeline alleviates dry mouth symptoms by stimulating secretion by the salivary glands. Cevimeline has a longer duration of salivation[3]. Cevimeline plays a part in Alzheimer’s disease. Cevimeline decreased Aβ (1–40) level in the cerebrospinal fluid (CSF) at 1 mg/kg without changing α-APPs in rabbit and significantly decreased CSF Aβ in AD patients.[4]
References:
1. F. B. Vivino, I. Al-Hashimi, Z. Khan, F. G. LeVeque, P. L. Salisbury, 3rd, T. K. Tran-Johnson, C. C. Muscoplat, M. Trivedi, B. Goldlust and S. C. Gallagher, Arch Intern Med 1999, 159, 174-181.
2. M. Ono, E. Takamura, K. Shinozaki, T. Tsumura, T. Hamano, Y. Yagi and K. Tsubota, Am J Ophthalmol 2004, 138, 6-17.
3. K. Ono, T. Inagaki, T. Iida, R. Hosokawa and K. Inenaga, J Med Invest 2009, 56 Suppl, 375.
4. A. Fisher, Z. Pittel, R. Haring, N. Bar-Ner, M. Kliger-Spatz, N. Natan, I. Egozi, H. Sonego, I. Marcovitch and R. Brandeis, J Mol Neurosci 2003, 20, 349-356.
- Epigoitrin
Catalog No.:BCN6278
CAS No.:1072-93-1
- AT-406 (SM-406)
Catalog No.:BCC1283
CAS No.:1071992-99-8
- Deoxyflindissone
Catalog No.:BCN7268
CAS No.:107176-31-8
- MAC13243
Catalog No.:BCC1727
CAS No.:1071638-38-4
- Pyrocincholic acid methyl ester
Catalog No.:BCN5873
CAS No.:107160-24-7
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- 8,9-Dihydroxy-10-isobutyryloxythymol
Catalog No.:BCN7974
CAS No.:107109-97-7
- Adipic dihydrazide
Catalog No.:BCC8810
CAS No.:1071-93-8
- Apo-12'-Lycopenal
Catalog No.:BCC8298
CAS No.:1071-52-9
- EIT hydrobromide
Catalog No.:BCC6824
CAS No.:1071-37-0
- O-Phosphorylethanolamine
Catalog No.:BCN1759
CAS No.:1071-23-4
- Amyloid Beta-Peptide (12-28) (human)
Catalog No.:BCC1044
CAS No.:107015-83-8
- 2-[(1S)-2-Formyl-1,3,3-trimethylcyclohexyl]-4-hydroxy-5-propan-2-ylbenzaldehyde
Catalog No.:BCN3584
CAS No.:1072444-55-3
- NPPB
Catalog No.:BCC6711
CAS No.:107254-86-4
- Baogongteng C
Catalog No.:BCN1873
CAS No.:107259-50-7
- Carasinol D
Catalog No.:BCN8228
CAS No.:1072797-66-0
- MLN2238
Catalog No.:BCC2092
CAS No.:1072833-77-2
- SR-3677
Catalog No.:BCC4302
CAS No.:1072959-67-1
- Defactinib
Catalog No.:BCC5494
CAS No.:1073154-85-4
- Demethylzeylasteral
Catalog No.:BCN2282
CAS No.:107316-88-1
- LDC000067
Catalog No.:BCC5452
CAS No.:1073485-20-7
- Cleroindicin B
Catalog No.:BCN5874
CAS No.:107389-91-3
- Garcinone D
Catalog No.:BCN2526
CAS No.:107390-08-9
- CD 1530
Catalog No.:BCC7406
CAS No.:107430-66-0
Comparison of the discontinuation rates and side-effect profiles of pilocarpine and cevimeline for xerostomia in primary Sjogren's syndrome.[Pubmed:25065774]
Clin Exp Rheumatol. 2014 Jul-Aug;32(4):575-7. Epub 2014 Jul 23.
OBJECTIVES: There are currently no head-to-head comparisons of sialagogues for Primary Sjogren's syndrome (pSS). We compared the tolerability and side effect profile of pilocarpine and Cevimeline in patients with pSS and determined clinical, laboratory and pathological variables associated with therapeutic failure. METHODS: We retrospectively reviewed the use of pilocarpine and Cevimeline in 118 patients with pSS who fulfilled the 2002 American European Consensus Group criteria in a University-based setting. Clinical, laboratory and pathological baseline variables were collected. Failure of therapy was defined as the clinician or patient's decision to stop treatment either due to lack of efficacy or side effects. RESULTS: Cevimeline was associated with lower failure rates compared to pilocarpine among first-time users: 27% vs. 47% (p=0.02), and all users: 32% vs. 61% (p<0.001). Severe sweating was the most frequent side effect leading to cessation of therapy and occurred more frequently in pilocarpine (25%) than Cevimeline (11%) users (p=0.02). Patients who previously failed one secretagogue were less likely to discontinue treatment with the other agent, 52% of first-time users vs. 27% of second-time users (p=0.004). Only ANA positivity was associated with failure: [59% vs. 38%] (p=0.03). CONCLUSIONS: pSS patients were more likely to continue Cevimeline than pilocarpine long-term due to fewer reported side effects with Cevimeline. Therapeutic failure of one secretagogue did not predict similar results with the other since second time users were more likely to continue long-term treatment.
Efficacy of cevimeline vs. pilocarpine in the secretion of saliva: a pilot study.[Pubmed:23600983]
Spec Care Dentist. 2013 May-Jun;33(3):123-7.
OBJECTIVES: To determine the efficacy and compare the side-effects of Cevimeline and pilocarpine in the secretion of saliva in patients with xerostomia. METHODS: A randomized, cross-over, double blind study was designed. Fifteen patients with diagnosis of xerostomia were assigned to take either 5 mg of pilocarpine or 30 mg of Cevimeline three times a day for four weeks. Salivary flow rates were measured during the initial baseline, first and second month appointments. Statistical analysis was carried out with ANOVA and post hoc t-tests. RESULTS: Twelve patients completed both medication treatments. Although both medications proved to increase salivary secretion, there was no significant difference between pilocarpine and Cevimeline. Also, the perceived side-effects between the two medications were similar. CONCLUSION: Both medications increased the secretion of saliva at the end of four weeks. However, there was a slightly higher increment in saliva with pilocarpine. However, the difference was not statistically significant.
An immunohistochemistry-based study on aquaporin (AQP)-1, 3, 4, 5 and 8 in the parotid glands, submandibular glands and sublingual glands of Sjogren's syndrome mouse models chronically administered cevimeline.[Pubmed:23925155]
Kurume Med J. 2013;60(1):7-19. Epub 2013 Aug 7.
Cevimeline is a muscarinic agonist that promotes saliva secretion and is used to treat Sjogren's syndrome (SS), an autoimmune disorder in which the exocrine glands that produce saliva are destroyed. Cevimeline is thought to affect the composition of saliva in part by regulating the localization of aquaporins (AQPs). In this study, we investigated the effects of chronic Cevimeline administration in the salivary glands of SS mice on the immunohistochemical localization of aquaporin (AQP)-1, 3, 4, 5 and 8. We used Cevimeline-untreated SS mice, treated SS mice, discontinued SS mice and untreated normal mice. AQP-5 was found in the apical and lateral membranes of acinar cells in the parotid and submandibular glands of Cevimeline-treated SS mice and untreated normal mice. Saliva secretion and AQP-5 localization were sustained in SS mice who were chronically administered Cevimeline and at four weeks after discontinuation. Unlike AQP-5, the localization of AQP-1, 3, 4 and 8 were not affected by Cevimeline administration. Our findings demonstrated that administration of Cevimeline maintains the proper localization of AQP-5 in the acinar cells of the salivary gland, which may promote salivation in chronically treated SS mice. Clinically, this suggests that chronic Cevimeline administration may be useful therapeutically for SS patients suffering from a decrease in saliva secretion by improving the disordered AQP-5 localization.
Structural characterization of cevimeline and its trans-impurity by single crystal XRD.[Pubmed:26609680]
J Pharm Biomed Anal. 2016 Jan 25;118:404-409.
Cevimeline is muscarinic receptor agonist which increases secretion of exocrine glands. Cevimeline base is a liquid (m.p. 20-25 degrees C) at ambient conditions, therefore its pharmaceutical formulation as a solid hydrochloride hemihydrate has been developed. The synthesis of Cevimeline yields its cis- and trans-isomers and only the cis-isomer is recognized as the API and used in the finished formulation. In this study structural and physicochemical investigations of hydrochloride hemihydrates of cis- and trans-Cevimelines have been performed. Single crystal X-ray analyses of both cis- and trans-isomers of Cevimeline are reported here for the first time. It was found that the cis-isomer, the API, has less dense crystal packing, lower melting point and higher solubility in comparison to the trans-isomer.


