DemethylzeylasteralCAS# 107316-88-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 107316-88-1 | SDF | Download SDF |
| PubChem ID | 10322911 | Appearance | Yellow powder |
| Formula | C29H36O6 | M.Wt | 480.60 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Synonyms | TZ 93 | ||
| Solubility | DMSO : 250 mg/mL (520.19 mM; Need ultrasonic) | ||
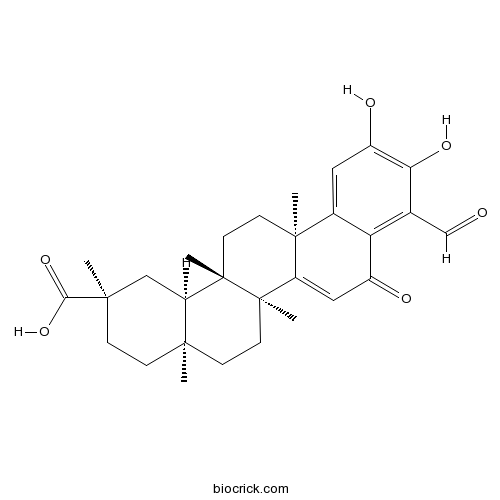
| Chemical Name | (2R,4aS,6aR,6aS,14aS,14bR)-9-formyl-10,11-dihydroxy-2,4a,6a,6a,14a-pentamethyl-8-oxo-1,3,4,5,6,13,14,14b-octahydropicene-2-carboxylic acid | ||
| SMILES | CC12CCC(CC1C3(CCC4(C5=CC(=C(C(=C5C(=O)C=C4C3(CC2)C)C=O)O)O)C)C)(C)C(=O)O | ||
| Standard InChIKey | ZDZSFWLPCFRASW-CPISFEQASA-N | ||
| Standard InChI | InChI=1S/C29H36O6/c1-25-6-7-26(2,24(34)35)14-21(25)29(5)11-9-27(3)17-12-19(32)23(33)16(15-30)22(17)18(31)13-20(27)28(29,4)10-8-25/h12-13,15,21,32-33H,6-11,14H2,1-5H3,(H,34,35)/t21-,25-,26-,27+,28-,29+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Demethylzeylasteral exhibits strong inhibition towards UDP-glucuronosyltransferase (UGT) 1A6 and 2B7, and the inhibition kinetic parameters (Ki) are calculated to be 0.6 uM and 17.3 uM for UGT1A6 and UGT2B7, respectively. Demethylzeylasteral has antimicrobial, strong immunosuppressive, and antifertility activities; it concentration-dependently and in a partially reversible manner can inhibit the Ca(2+) current in spermatogenic cells with an IC(50) of 8.8 microg/ml, and it also can inhibit significantly the sperm acrosome reaction initiated by progesterone. |
| Targets | Antifection | Immunology & Inflammation related | UGT1A6 | UGT2B7 | Calcium Channel |
| In vitro | Demethylzeylasteral exhibits dose-dependent inhibitory behaviour towards estradiol glucuronidation.[Pubmed: 23807732]Eur J Drug Metab Pharmacokinet. 2014 Jun;39(2):99-102.The disturbance of estradiol level might induce the occurence of some diseases, including cancer. Estradiol is mainly metabolized through the conjugation reactions, including the sulfation and glucuronidation reactions. The present study tried to evaluate the inhibition of estradiol glucuronidation by the major ingredients of Tripterygium wilfordii Hook F. Demethylzeylasteral.
Effects of demethylzeylasteral and celastrol on spermatogenic cell Ca2+ channels and progesterone-induced sperm acrosome reaction.[Pubmed: 12600689]Eur J Pharmacol. 2003 Mar 7;464(1):9-15.The male antifertility effect of a water-chloroform extract of Tripterygium wilfordii Hook. f. (GTW) and several monomers isolated from GTW has attracted worldwide interest.
Antimicrobial activity of 6-oxophenolic triterpenoids. Mode of action against Bacillus subtilis.[Pubmed: 15856406 ]Planta Med. 2005 Apr;71(4):313-9.Zeylasteral and Demethylzeylasteral are 6-oxophenolic triterpenoids isolated from the root of Maytenus blepharodes, which have antimicrobial activity against Gram-positive bacteria and the yeast Candida albicans.
|
| Kinase Assay | Demethylzeylasteral exhibits strong inhibition towards UDP-glucuronosyltransferase (UGT) 1A6 and 2B7.[Pubmed: 22874791]Molecules. 2012 Aug 8;17(8):9469-75.Inhibition of UDP-glucuronosyltransferase (UGT) isoforms can result in severe clinical results, including clinical drug-drug interactions (DDI) and metabolic disorders of endogenous substances. The present study aims to investigate the inhibition of Demethylzeylasteral (an important active component isolated from Tripterygium wilfordii Hook F.) towards three important UGT isoforms UGT1A6, UGT1A9 and UGT2B7.
|
| Animal Research | Immunosuppressive effects of demethylzeylasteral in a rat kidney transplantation model.[Pubmed: 19383554]Int Immunopharmacol. 2009 Jul;9(7-8):996-1001.In this study, we examined the immunosuppressive activity of Demethylzeylasteral (T-96), isolated from the traditional Chinese herbal medicine,
Tripterygium wilfordii Hook f. Its immunosuppressive effect was investigated using mouse splenocytes in vitro, and in an in vivo rat kidney transplant model. |

Demethylzeylasteral Dilution Calculator

Demethylzeylasteral Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0807 mL | 10.4037 mL | 20.8073 mL | 41.6146 mL | 52.0183 mL |
| 5 mM | 0.4161 mL | 2.0807 mL | 4.1615 mL | 8.3229 mL | 10.4037 mL |
| 10 mM | 0.2081 mL | 1.0404 mL | 2.0807 mL | 4.1615 mL | 5.2018 mL |
| 50 mM | 0.0416 mL | 0.2081 mL | 0.4161 mL | 0.8323 mL | 1.0404 mL |
| 100 mM | 0.0208 mL | 0.104 mL | 0.2081 mL | 0.4161 mL | 0.5202 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Defactinib
Catalog No.:BCC5494
CAS No.:1073154-85-4
- SR-3677
Catalog No.:BCC4302
CAS No.:1072959-67-1
- MLN2238
Catalog No.:BCC2092
CAS No.:1072833-77-2
- Carasinol D
Catalog No.:BCN8228
CAS No.:1072797-66-0
- Baogongteng C
Catalog No.:BCN1873
CAS No.:107259-50-7
- NPPB
Catalog No.:BCC6711
CAS No.:107254-86-4
- 2-[(1S)-2-Formyl-1,3,3-trimethylcyclohexyl]-4-hydroxy-5-propan-2-ylbenzaldehyde
Catalog No.:BCN3584
CAS No.:1072444-55-3
- Cevimeline
Catalog No.:BCC1470
CAS No.:107233-08-9
- Epigoitrin
Catalog No.:BCN6278
CAS No.:1072-93-1
- AT-406 (SM-406)
Catalog No.:BCC1283
CAS No.:1071992-99-8
- Deoxyflindissone
Catalog No.:BCN7268
CAS No.:107176-31-8
- MAC13243
Catalog No.:BCC1727
CAS No.:1071638-38-4
- LDC000067
Catalog No.:BCC5452
CAS No.:1073485-20-7
- Cleroindicin B
Catalog No.:BCN5874
CAS No.:107389-91-3
- Garcinone D
Catalog No.:BCN2526
CAS No.:107390-08-9
- CD 1530
Catalog No.:BCC7406
CAS No.:107430-66-0
- Ginkgolide J
Catalog No.:BCN5939
CAS No.:107438-79-9
- Bisabola-2,10-diene-1,9-dione
Catalog No.:BCN7269
CAS No.:107439-25-8
- Glucagon-like peptide 1 (7-36) amide (human, rat)
Catalog No.:BCC7258
CAS No.:107444-51-9
- Omadacycline tosylate
Catalog No.:BCC5136
CAS No.:1075240-43-5
- Anwulignan
Catalog No.:BCN5362
CAS No.:107534-93-0
- 3'-Hydroxy-3,9-dihydroeucomin
Catalog No.:BCN5875
CAS No.:107585-75-1
- 4-Demethyl-3,9-dihydroeucomin
Catalog No.:BCN5876
CAS No.:107585-77-3
- 4-Hydroxycoumarin
Catalog No.:BCN2561
CAS No.:1076-38-6
Antimicrobial activity of 6-oxophenolic triterpenoids. Mode of action against Bacillus subtilis.[Pubmed:15856406]
Planta Med. 2005 Apr;71(4):313-9.
Zeylasteral and Demethylzeylasteral are 6-oxophenolic triterpenoids isolated from the root of Maytenus blepharodes, which have antimicrobial activity against Gram-positive bacteria and the yeast Candida albicans. The time-kill curves for zeylasteral and Demethylzeylasteral at concentrations twice their MICs, against Bacillus subtilis showed that the colony forming units were reduced in 3-log10 and > 4-log10 respectively. This reduction was dependent on inoculum size and the growth phase of cells, and was greater when the compounds were incorporated in the exponential phase, indicating a bacteriolytic effect. Treatment with both agents, particularly with zeylasteral (20 microg/mL) caused a reduction of optical density at 550 nm. With regard to the synthesis of DNA, RNA, protein and cell wall, the compounds exhibited the fastest inhibition against cell wall synthesis. Thus, the predisposition to lysis, the morphological changes seen by microscopy, and the complete inhibition in the incorporation the N-acetyl-d-[1 - 14C]glucosamine, suggest that the phenolic compounds compromise the cell wall synthesis and/or cytoplasmic membrane.
Demethylzeylasteral exhibits strong inhibition towards UDP-glucuronosyltransferase (UGT) 1A6 and 2B7.[Pubmed:22874791]
Molecules. 2012 Aug 8;17(8):9469-75.
Inhibition of UDP-glucuronosyltransferase (UGT) isoforms can result in severe clinical results, including clinical drug-drug interactions (DDI) and metabolic disorders of endogenous substances. The present study aims to investigate the inhibition of Demethylzeylasteral (an important active component isolated from Tripterygium wilfordii Hook F.) towards three important UGT isoforms UGT1A6, UGT1A9 and UGT2B7. The results showed that 100 muM of Demethylzeylasteral exhibited strong inhibition towards UGT1A6 and UGT2B7, with negligible influence towards UGT1A9. Furthermore, Dixon and Lineweaver-Burk plots showed the inhibition of UGT1A6 and UGT2B7 by Demethylzeylasteral was best fit to competitive inhibition, and the inhibition kinetic parameters (Ki) were calculated to be 0.6 muM and 17.3 muM for UGT1A6 and UGT2B7, respectively. This kind of inhibitory effect need much attention when Demethylzeylasteral and demethylzeyasteral-containing herbs (e.g., Tripterygium wilfordii Hook F.) were co-administered with the drugs mainly undergoing UGT1A6, UGT2B7-catalyzed metabolism. However, when extrapolating the in vivo clinical results using our present in vitro data, many complex factors might affect final results, including the contribution of UGT1A6 and UGT2B7 to the metabolism of compounds, and the herbal or patients' factors affecting the in vivo concentration of Demethylzeylasteral.
Effects of demethylzeylasteral and celastrol on spermatogenic cell Ca2+ channels and progesterone-induced sperm acrosome reaction.[Pubmed:12600689]
Eur J Pharmacol. 2003 Mar 7;464(1):9-15.
The male antifertility effect of a water-chloroform extract of Tripterygium wilfordii Hook. f. (GTW) and several monomers isolated from GTW has attracted worldwide interest. In the present study, the effects of two isolated monomers from GTW, Demethylzeylasteral and celastrol, on the Ca(2+) channels in mouse spermatogenic cells and on the sperm acrosome reaction were investigated by whole-cell patch-clamp recording and chlortetracycline staining methods, respectively. The results showed that Demethylzeylasteral concentration-dependently and in a partially reversible manner inhibited the Ca(2+) current in spermatogenic cells with an IC(50) of 8.8 microg/ml. Celastrol decreased the Ca(2+) current in the cells time-dependently and irreversibly. The changes in the activation and inactivation time constants of Ca(2+) currents after application of these two compounds were also examined. Demethylzeylasteral increased both activation and inactivation time constants of Ca(2+) currents, and celastrol had no significant effect on them. The two compounds also inhibited significantly the sperm acrosome reaction initiated by progesterone. These data suggest that inhibition of Ca(2+) currents could be responsible for the antifertility activity of these compounds.
Immunosuppressive effects of demethylzeylasteral in a rat kidney transplantation model.[Pubmed:19383554]
Int Immunopharmacol. 2009 Jul;9(7-8):996-1001.
In this study, we examined the immunosuppressive activity of Demethylzeylasteral (T-96), isolated from the traditional Chinese herbal medicine, Tripterygium wilfordii Hook f. Its immunosuppressive effect was investigated using mouse splenocytes in vitro, and in an in vivo rat kidney transplant model. T-96 inhibited mouse splenocyte proliferation in a dose dependent manner. In the rat kidney transplant study, rats were randomly divided into eight groups following kidney transplantation, and different doses of T-96 or cyclosporin A (CsA) were administered to each group. T-96 alone at doses of 10 or 20 mg/kg/day significantly prolonged the survival of kidney-transplanted rats, compared with transplanted but untreated control rats. A combination of T-96 and prednisone also significantly prolonged survival: 10 mg/kg/day T-96 with 10 mg/kg/day prednisone increased the survival time to 31.8+/-6.5 days. Moreover, the combination of T-96 and prednisone was also effective in suppressing rejection of rat transplanted kidneys. These results demonstrate the strong immunosuppressive activity of T-96 and suggest a possible clinical use for T-96 as an immunosuppressive agent in the fields of organ transplantation and autoimmune disorders.
Demethylzeylasteral exhibits dose-dependent inhibitory behaviour towards estradiol glucuronidation.[Pubmed:23807732]
Eur J Drug Metab Pharmacokinet. 2014 Jun;39(2):99-102.
The disturbance of estradiol level might induce the occurence of some diseases, including cancer. Estradiol is mainly metabolized through the conjugation reactions, including the sulfation and glucuronidation reactions. The present study tried to evaluate the inhibition of estradiol glucuronidation by the major ingredients of Tripterygium wilfordii Hook F. Demethylzeylasteral. Selective ion monitoring at negative ion mode ([M(+) H(-)] = 447) was employed to monitor the two glucuronides of estradiol. The reaction rate was determined through comparison of peak area of these two glucuronides. Lineweaver-Burk plot and Dixon plot were utilized to determine the inhibition kinetic type, and the inhibition kinetic parameters (K i) were calculated using the second plot. Competitive inhibition of Demethylzeylasteral towards the formation of two glucuronides of estradiol was demonstrated, and the K i values were calculated to be 453.3 and 110.9 muM, respectively. All these results will remind us the risk of elevated serum concentrations of estradiol due to the inhibition of estradiol glucuronidation by Demethylzeylasteral.


