Garcinone DCAS# 107390-08-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 107390-08-9 | SDF | Download SDF |
| PubChem ID | 5495926 | Appearance | Yellow powder |
| Formula | C24H28O7 | M.Wt | 428.48 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in acetone, chloroform, diethyl ether, ethyl acetate and methanol; practically insoluble in water | ||
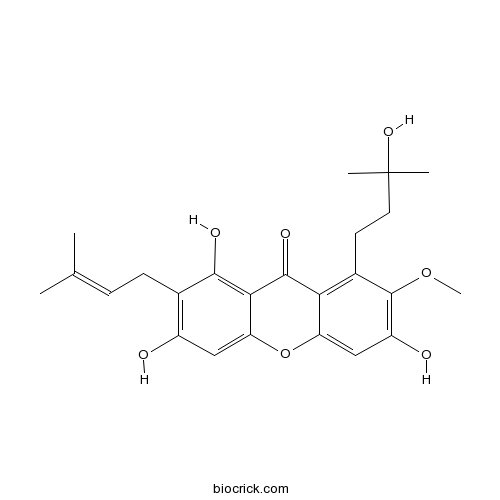
| Chemical Name | 1,3,6-trihydroxy-8-(3-hydroxy-3-methylbutyl)-7-methoxy-2-(3-methylbut-2-enyl)xanthen-9-one | ||
| SMILES | CC(=CCC1=C(C=C2C(=C1O)C(=O)C3=C(C(=C(C=C3O2)O)OC)CCC(C)(C)O)O)C | ||
| Standard InChIKey | TYALNCRUIKOKGP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H28O7/c1-12(2)6-7-13-15(25)10-18-20(21(13)27)22(28)19-14(8-9-24(3,4)29)23(30-5)16(26)11-17(19)31-18/h6,10-11,25-27,29H,7-9H2,1-5H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Garcinone D shows significant cytotoxicity against the CEM-SS cell line, with IC(50) value of 3.2 microg/ml. 2. Garcinone D inhibits p65 activation with IC50 values of 3.2 microM. 3. Garcinone D exhibits dose-dependent enzyme-based microsomal aromatase inhibitory activity. |
| Targets | p65 | NF-kB |

Garcinone D Dilution Calculator

Garcinone D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3338 mL | 11.6692 mL | 23.3383 mL | 46.6766 mL | 58.3458 mL |
| 5 mM | 0.4668 mL | 2.3338 mL | 4.6677 mL | 9.3353 mL | 11.6692 mL |
| 10 mM | 0.2334 mL | 1.1669 mL | 2.3338 mL | 4.6677 mL | 5.8346 mL |
| 50 mM | 0.0467 mL | 0.2334 mL | 0.4668 mL | 0.9335 mL | 1.1669 mL |
| 100 mM | 0.0233 mL | 0.1167 mL | 0.2334 mL | 0.4668 mL | 0.5835 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cleroindicin B
Catalog No.:BCN5874
CAS No.:107389-91-3
- LDC000067
Catalog No.:BCC5452
CAS No.:1073485-20-7
- Demethylzeylasteral
Catalog No.:BCN2282
CAS No.:107316-88-1
- Defactinib
Catalog No.:BCC5494
CAS No.:1073154-85-4
- SR-3677
Catalog No.:BCC4302
CAS No.:1072959-67-1
- MLN2238
Catalog No.:BCC2092
CAS No.:1072833-77-2
- Carasinol D
Catalog No.:BCN8228
CAS No.:1072797-66-0
- Baogongteng C
Catalog No.:BCN1873
CAS No.:107259-50-7
- NPPB
Catalog No.:BCC6711
CAS No.:107254-86-4
- 2-[(1S)-2-Formyl-1,3,3-trimethylcyclohexyl]-4-hydroxy-5-propan-2-ylbenzaldehyde
Catalog No.:BCN3584
CAS No.:1072444-55-3
- Cevimeline
Catalog No.:BCC1470
CAS No.:107233-08-9
- Epigoitrin
Catalog No.:BCN6278
CAS No.:1072-93-1
- CD 1530
Catalog No.:BCC7406
CAS No.:107430-66-0
- Ginkgolide J
Catalog No.:BCN5939
CAS No.:107438-79-9
- Bisabola-2,10-diene-1,9-dione
Catalog No.:BCN7269
CAS No.:107439-25-8
- Glucagon-like peptide 1 (7-36) amide (human, rat)
Catalog No.:BCC7258
CAS No.:107444-51-9
- Omadacycline tosylate
Catalog No.:BCC5136
CAS No.:1075240-43-5
- Anwulignan
Catalog No.:BCN5362
CAS No.:107534-93-0
- 3'-Hydroxy-3,9-dihydroeucomin
Catalog No.:BCN5875
CAS No.:107585-75-1
- 4-Demethyl-3,9-dihydroeucomin
Catalog No.:BCN5876
CAS No.:107585-77-3
- 4-Hydroxycoumarin
Catalog No.:BCN2561
CAS No.:1076-38-6
- PF 998425
Catalog No.:BCC7811
CAS No.:1076225-27-8
- Dehydroformouregine
Catalog No.:BCN4054
CAS No.:107633-69-2
- Erycibelline
Catalog No.:BCN1876
CAS No.:107633-95-4
Garcinia mangostana: a source of potential anti-cancer lead compounds against CEM-SS cell line.[Pubmed:18464091]
J Asian Nat Prod Res. 2008 May-Jun;10(5-6):475-9.
Our current interest in searching for natural anti-cancer lead compounds from plants has led us to the discovery that the stem and roots of Garcinia mangostana can be a source of such compounds. The stem furnished 2,8-dihydroxy-6-methoxy-5-(3-methylbut-2-enyl)-xanthone (1), which is a new xanthone. Meanwhile, the root bark of the plant furnished six xanthones, namely alpha-mangostin (2), beta-mangostin (3), gamma-mangostin (4), Garcinone D (5), mangostanol (6), and gartanin (7). The hexane and chloroform extracts of the root bark of G. mangostana as well as the hexane extract of the stem bark were found to be active against the CEM-SS cell line. gamma-Mangostin (4) showed good activity with a very low IC(50) value of 4.7 microg/ml, while alpha-mangostin (2), mangostanol (6), and Garcinone D (5) showed significant activities with IC(50) values of 5.5, 9.6, and 3.2 microg/ml, respectively. This is the first report on the cytotoxicity of the extracts of the stem and root bark of G. mangostana and of alpha-mangostin, mangostanol, and Garcinone D against the CEM-SS cell line.
Xanthones from the botanical dietary supplement mangosteen (Garcinia mangostana) with aromatase inhibitory activity.[Pubmed:18558747]
J Nat Prod. 2008 Jul;71(7):1161-6.
Twelve xanthone constituents of the botanical dietary supplement mangosteen (the pericarp of Garcinia mangostana) were screened using a noncellular, enzyme-based microsomal aromatase inhibition assay. Of these compounds, Garcinone D (3), garcinone E (5), alpha-mangostin (8), and gamma-mangostin (9) exhibited dose-dependent inhibitory activity. In a follow-up cell-based assay using SK-BR-3 breast cancer cells that express high levels of aromatase, the most potent of these four xanthones was gamma-mangostin (9). Because xanthones may be consumed in substantial amounts from commercially available mangosteen products, the consequences of frequent intake of mangosteen botanical dietary supplements require further investigation to determine their possible role in breast cancer chemoprevention.
Cytotoxic xanthone constituents of the stem bark of Garcinia mangostana (mangosteen).[Pubmed:19839614]
J Nat Prod. 2009 Nov;72(11):2028-31.
Bioassay-guided fractionation of a chloroform-soluble extract of Garcinia mangostana stem bark, using the HT-29 human colon cancer cell line and an enzyme-based ELISA NF-kappaB assay, led to the isolation of a new xanthone, 11-hydroxy-3-O-methyl-1-isomangostin (1). The structure of 1 was elucidated by spectroscopic data analysis. In addition, 10 other known compounds, 11-hydroxy-1-isomangostin (2), 11alpha-mangostanin (3), 3-isomangostin (4), alpha-mangostin (5), beta-mangostin (6), Garcinone D (7), 9-hydroxycalabaxanthone (8), 8-deoxygartanin (9), gartanin (10), and cratoxyxanthone (11), were isolated. Compounds 4-8 exhibited cytotoxicity against the HT-29 cell line with ED50 values of 4.9, 1.7, 1.7, 2.3, and 9.1 microM, respectively. In an ELISA NF-kappaB assay, compounds 5-7, 9, and 10 inhibited p65 activation with IC50 values of 15.9, 12.1, 3.2, 11.3, and 19.0 microM, respectively, and 6 showed p50 inhibitory activity with an IC50 value of 7.5 microM. Alpha-mangostin (5) was further tested in an in vivo hollow fiber assay, using HT-29, LNCaP, and MCF-7 cells, but it was found to be inactive at the highest dose tested (20 mg/kg).


