LDC000067CDK9 inhibitor, novel and highly specific CAS# 1073485-20-7 |
- Losartan
Catalog No.:BCC4090
CAS No.:114798-26-4
- Eprosartan Mesylate
Catalog No.:BCC4658
CAS No.:144143-96-4
- AHU-377(Sacubitril)
Catalog No.:BCC4088
CAS No.:149709-62-6
- AVE 0991
Catalog No.:BCC4032
CAS No.:304462-19-9
- AVE 0991 sodium salt
Catalog No.:BCC4222
CAS No.:306288-04-0
- Azilsartan medoxomil monopotassium
Catalog No.:BCC4089
CAS No.:863031-24-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1073485-20-7 | SDF | Download SDF |
| PubChem ID | 25104564 | Appearance | Powder |
| Formula | C18H18N4O3S | M.Wt | 370.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | LDC067 | ||
| Solubility | DMSO : ≥ 47 mg/mL (126.88 mM) *"≥" means soluble, but saturation unknown. | ||
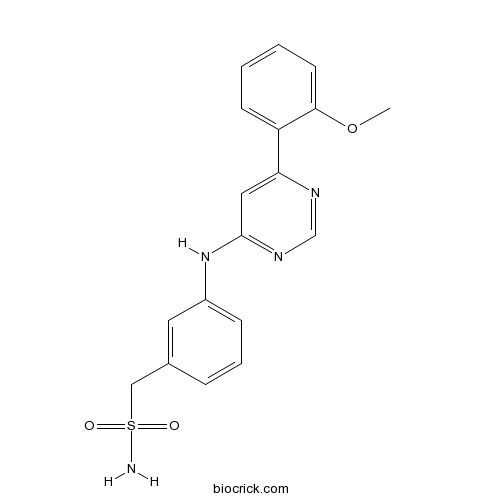
| Chemical Name | [3-[[6-(2-methoxyphenyl)pyrimidin-4-yl]amino]phenyl]methanesulfonamide | ||
| SMILES | COC1=CC=CC=C1C2=CC(=NC=N2)NC3=CC=CC(=C3)CS(=O)(=O)N | ||
| Standard InChIKey | GGQCIOOSELPMBB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H18N4O3S/c1-25-17-8-3-2-7-15(17)16-10-18(21-12-20-16)22-14-6-4-5-13(9-14)11-26(19,23)24/h2-10,12H,11H2,1H3,(H2,19,23,24)(H,20,21,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | LDC000067 is a highly specific CDK9 inhibitor with an IC50 value of 44±10 nM in vitro.In Vitro:The selectivity of LDC000067 for CDK9 over other CDKs exceeds that of the known inhibitors flavopiridol and DRB. LDC000067 displayed 55/125/210/ >227/ >227-fold selectivity for CDK9 versus CDK2/1/4/6/7. LDC000067 inhibits in vitro transcription in an ATP-competitive and dose-dependent manner. Gene expression profiling of cells treated with LDC000067 demonstrates a selective reduction of short-lived mRNAs, including important regulators of proliferation and apoptosis[1]. References: | |||||
| Kinase experiment [1]: | |
| Inhibitory activities | Kinase inhibition by LDC000067 was measured in a radio metric assay, which directly measured kinase catalytic activity towards a specific substrate. Briefly, 10 μM LDC000067 or DMSO as solvent control, were added to base reaction buffer (10 mM MgCl2, 1 mM EDTA, 20 mM HEPES pH 7.5, 2 mM DTT, 0.02 mg·mL-1 BSA, 0.1 mM Na3VO4, 0.02% Brij35, 1% DMSO), containing cofactors and substrates, which were required by the individual kinase. 10 μCi of [γ-33P]-APP (10mCi·mL-1, 3000Ci·mmol-1, Perkin Elmer) was added to the reaction mixture. And such kinase reaction incubated for 120min at room temperature. Reactions were found on P81 ion exchange paper, and filters generally washed in 0.75% phosphoric acid before radiometric quantification. Each protein kinase was measured in duplicate, and its catalytic activity expressed as residual kinase activity, which shows the percentage of average substrate phophorylation in contrast with the solvent control reaction. |
| Cell experiment [1]: | |
| Cell lines | HEK293T cells, THP1 cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | Competitive kinase binding displacement assay: 90 min.DNA microarray analysis assay: 90 min. |
| Applications | LDC000067 inhibits CDK9 with an IC50 value of 44(10 nM, and its selectivity for CDK9 over other CDKs is in the range of 55-fold to over 230-fold, especially higher selectivity in an ATP-competitive kinase binding assay. Besides, effects of LDC000067 in whole cells contain induction of the tumour suppressor protein p53 and apoptosis. Moreover, gene expression profiling of cells treated with LDC000067 demonstrate selective reduction of short-lived mRNAs, which encode regulators of proliferation and apoptosis, such as MCL1 and MYC. |
| References: [1]. T K Albert1, C Rigault1, J Eickhoff, K Baumgart, C Antrecht1, B Klebl, G Mittler and M Meisterernst. Characterization of molecular and cellular functions of the cyclin-dependent kinase CDK9 using a novel specific inhibitor. British Journal of Pharmacology. 2014, 171: 55–68. | |

LDC000067 Dilution Calculator

LDC000067 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6996 mL | 13.4978 mL | 26.9957 mL | 53.9913 mL | 67.4891 mL |
| 5 mM | 0.5399 mL | 2.6996 mL | 5.3991 mL | 10.7983 mL | 13.4978 mL |
| 10 mM | 0.27 mL | 1.3498 mL | 2.6996 mL | 5.3991 mL | 6.7489 mL |
| 50 mM | 0.054 mL | 0.27 mL | 0.5399 mL | 1.0798 mL | 1.3498 mL |
| 100 mM | 0.027 mL | 0.135 mL | 0.27 mL | 0.5399 mL | 0.6749 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LDC000067 (LDC067) is a novel specific inhibitor of CDK9 with IC50 value of 44 ± 10 nM [1].
Cyclin-dependent kinase 9 (CDK9) is a cyclin-dependent kinase. CDK9 and cyclin T form the positive transcription elongation factor b (P-TEFb) complex for RNA polymerase II and functions by phosphorylating the C-terminal domain of the largest subunit of RNA polymerase II [1].
LDC000067 (LDC067) is a novel and highly specific CDK9 inhibitor. LDC000067 exhibited selectivity for CDK9 over other CDKs in the range of 55-fold (vs. CDK2) to over 230-fold (vs. CDK6 and CDK7). LDC067 also inhibited transcription in a dose-dependent and ATP-competitive manner. In whole cells, LDC000067 induced the tumor suppressor protein p53 activation and apoptosis. LDC000067 also selectively reduced short-lived mRNAs, including those that encode regulators of apoptosis and proliferation such as MYC and MCL1 [1].
Reference:
[1]. Albert TK, Rigault C, Eickhoff J, et al. Characterization of molecular and cellular functions of the cyclin-dependent kinase CDK9 using a novel specific inhibitor. Br J Pharmacol, 2014, 171(1): 55-68.
- Demethylzeylasteral
Catalog No.:BCN2282
CAS No.:107316-88-1
- Defactinib
Catalog No.:BCC5494
CAS No.:1073154-85-4
- SR-3677
Catalog No.:BCC4302
CAS No.:1072959-67-1
- MLN2238
Catalog No.:BCC2092
CAS No.:1072833-77-2
- Carasinol D
Catalog No.:BCN8228
CAS No.:1072797-66-0
- Baogongteng C
Catalog No.:BCN1873
CAS No.:107259-50-7
- NPPB
Catalog No.:BCC6711
CAS No.:107254-86-4
- 2-[(1S)-2-Formyl-1,3,3-trimethylcyclohexyl]-4-hydroxy-5-propan-2-ylbenzaldehyde
Catalog No.:BCN3584
CAS No.:1072444-55-3
- Cevimeline
Catalog No.:BCC1470
CAS No.:107233-08-9
- Epigoitrin
Catalog No.:BCN6278
CAS No.:1072-93-1
- AT-406 (SM-406)
Catalog No.:BCC1283
CAS No.:1071992-99-8
- Deoxyflindissone
Catalog No.:BCN7268
CAS No.:107176-31-8
- Cleroindicin B
Catalog No.:BCN5874
CAS No.:107389-91-3
- Garcinone D
Catalog No.:BCN2526
CAS No.:107390-08-9
- CD 1530
Catalog No.:BCC7406
CAS No.:107430-66-0
- Ginkgolide J
Catalog No.:BCN5939
CAS No.:107438-79-9
- Bisabola-2,10-diene-1,9-dione
Catalog No.:BCN7269
CAS No.:107439-25-8
- Glucagon-like peptide 1 (7-36) amide (human, rat)
Catalog No.:BCC7258
CAS No.:107444-51-9
- Omadacycline tosylate
Catalog No.:BCC5136
CAS No.:1075240-43-5
- Anwulignan
Catalog No.:BCN5362
CAS No.:107534-93-0
- 3'-Hydroxy-3,9-dihydroeucomin
Catalog No.:BCN5875
CAS No.:107585-75-1
- 4-Demethyl-3,9-dihydroeucomin
Catalog No.:BCN5876
CAS No.:107585-77-3
- 4-Hydroxycoumarin
Catalog No.:BCN2561
CAS No.:1076-38-6
- PF 998425
Catalog No.:BCC7811
CAS No.:1076225-27-8
Characterization of molecular and cellular functions of the cyclin-dependent kinase CDK9 using a novel specific inhibitor.[Pubmed:24102143]
Br J Pharmacol. 2014 Jan;171(1):55-68.
BACKGROUND AND PURPOSE: The cyclin-dependent kinase CDK9 is an important therapeutic target but currently available inhibitors exhibit low specificity and/or narrow therapeutic windows. Here we have used a new highly specific CDK9 inhibitor, LDC000067 to interrogate gene control mechanisms mediated by CDK9. EXPERIMENTAL APPROACH: The selectivity of LDC000067 was established in functional kinase assays. Functions of CDK9 in gene expression were assessed with in vitro transcription experiments, single gene analyses and genome-wide expression profiling. Cultures of mouse embryonic stem cells, HeLa cells, several cancer cell lines, along with cells from patients with acute myelogenous leukaemia were also used to investigate cellular responses to LDC000067. KEY RESULTS: The selectivity of LDC000067 for CDK9 over other CDKs exceeded that of the known inhibitors flavopiridol and DRB. LDC000067 inhibited in vitro transcription in an ATP-competitive and dose-dependent manner. Gene expression profiling of cells treated with LDC000067 demonstrated a selective reduction of short-lived mRNAs, including important regulators of proliferation and apoptosis. Analysis of de novo RNA synthesis suggested a wide ranging positive role of CDK9. At the molecular and cellular level, LDC000067 reproduced effects characteristic of CDK9 inhibition such as enhanced pausing of RNA polymerase II on genes and, most importantly, induction of apoptosis in cancer cells. CONCLUSIONS AND IMPLICATIONS: Our study provides a framework for the mechanistic understanding of cellular responses to CDK9 inhibition. LDC000067 represents a promising lead for the development of clinically useful, highly specific CDK9 inhibitors.


