AnwulignanCAS# 107534-93-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 107534-93-0 | SDF | Download SDF |
| PubChem ID | 10404245 | Appearance | White powder |
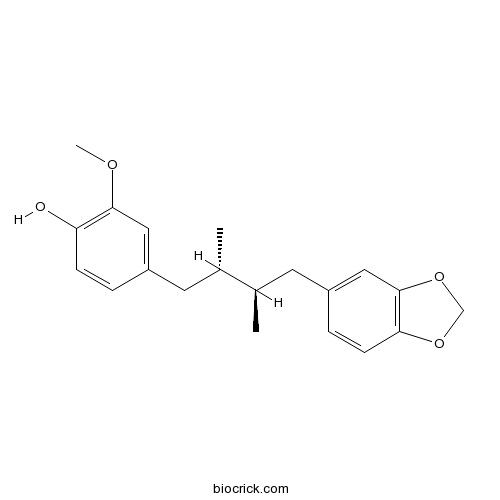
| Formula | C20H24O4 | M.Wt | 328.41 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Synonyms | (+)-Anwulignan; Anwuligan | ||
| Solubility | DMSO : ≥ 30 mg/mL (91.35 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-[(2S,3R)-4-(1,3-benzodioxol-5-yl)-2,3-dimethylbutyl]-2-methoxyphenol | ||
| SMILES | CC(CC1=CC2=C(C=C1)OCO2)C(C)CC3=CC(=C(C=C3)O)OC | ||
| Standard InChIKey | QDDILOVMGWUNGD-UONOGXRCSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Anwuligan has antimicrobial and anticariogenic activity against Streptococcus mutans and other streptococcus species. It also shows antioxidant, free radical scavenging, and neuroprotective activities. (+)-Anwulignan has inhibitory effects on platelets aggregation induced by adenosine diphosphate (ADP) and platelet activating factor(PAF) in vitro. |
| Targets | PAFR | Antifection |
| In vitro | Effects of heteroclitin D, schisanhenol and ( + )-anwulignan on platelet aggregation.[Reference: WebLink]Journal of Shanghai Medica, 2005, 32(4):467-70.
To investigate the effects of three lignans: heteroclitin D (HD), schisanhenol (SAL) and ( + )-Anwulignan(AN) with different skeletons from Schisandraceae medicinal plants on platelet aggregation. |
| Structure Identification | Se Pu. 2009 May;27(3):313-7.Selection of mobile phases for the determination of gamma-schisandrin and multi-active constituents in Schisandra chinensis and its preparations by high performance liquid chromatography.[Pubmed: 19803136]
|

Anwulignan Dilution Calculator

Anwulignan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.045 mL | 15.2249 mL | 30.4497 mL | 60.8995 mL | 76.1244 mL |
| 5 mM | 0.609 mL | 3.045 mL | 6.0899 mL | 12.1799 mL | 15.2249 mL |
| 10 mM | 0.3045 mL | 1.5225 mL | 3.045 mL | 6.0899 mL | 7.6124 mL |
| 50 mM | 0.0609 mL | 0.3045 mL | 0.609 mL | 1.218 mL | 1.5225 mL |
| 100 mM | 0.0304 mL | 0.1522 mL | 0.3045 mL | 0.609 mL | 0.7612 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Macelignan(Anwuligan) is a natural compound isolated from Myristica fragrans Houtt; possesses therapeutic potentials against neurodegenerative diseases with oxidative stress and neuroinflammation. IC50 value: Target: in vitro: Macelignan significantly attenuated the ROS production and neurotoxicity induced by glutamate in HT22 cell [1]. At 24 h of biofilm growth, S. mutans, A. viscosus and S. sanguis biofilms were reduced by up to 30%, 30% and 38%, respectively, after treatment with 10 microg/mL macelignan for 5 min [2]. Cisplatin-induced phosphorylation of c-Jun N-terminal kinase1/2 (JNK1/2) and extracellular signal-regulated kinase1/2 (ERK1/2) was abrogated by pretreatment with macelignan, however, that of p38 was not significantly affected [3]. in vivo: Macelignan attenuated the expression of phosphorylated c-Jun in cisplatin-treated mice [3]. Daily administration of macelignan reduced the spatial memory impairments induced by the chronic LPS infusions [4].
References:
[1]. Jin DQ, et al. Anti-oxidant and anti-inflammatory activities of macelignan in murine hippocampal cell line and primary culture of rat microglial cells. Biochem Biophys Res Commun. 2005 Jun 17;331(4):1264-9.
[2]. Yanti, et al. In vitro anti-biofilm activity of macelignan isolated from Myristica fragrans Houtt. against oral primary colonizer bacteria. Phytother Res. 2008 Mar;22(3):308-12.
[3]. Sohn JH, et al. Protective Effects of macelignan on cisplatin-induced hepatotoxicity is associated with JNK activation. Biol Pharm Bull. 2008 Feb;31(2):273-7.
[4]. Cui CA, et al. Macelignan attenuates LPS-induced inflammation and reduces LPS-induced spatial learning impairments in rats. Neurosci Lett. 2008 Dec 19;448(1):110-4.
- Omadacycline tosylate
Catalog No.:BCC5136
CAS No.:1075240-43-5
- Glucagon-like peptide 1 (7-36) amide (human, rat)
Catalog No.:BCC7258
CAS No.:107444-51-9
- Bisabola-2,10-diene-1,9-dione
Catalog No.:BCN7269
CAS No.:107439-25-8
- Ginkgolide J
Catalog No.:BCN5939
CAS No.:107438-79-9
- CD 1530
Catalog No.:BCC7406
CAS No.:107430-66-0
- Garcinone D
Catalog No.:BCN2526
CAS No.:107390-08-9
- Cleroindicin B
Catalog No.:BCN5874
CAS No.:107389-91-3
- LDC000067
Catalog No.:BCC5452
CAS No.:1073485-20-7
- Demethylzeylasteral
Catalog No.:BCN2282
CAS No.:107316-88-1
- Defactinib
Catalog No.:BCC5494
CAS No.:1073154-85-4
- SR-3677
Catalog No.:BCC4302
CAS No.:1072959-67-1
- MLN2238
Catalog No.:BCC2092
CAS No.:1072833-77-2
- 3'-Hydroxy-3,9-dihydroeucomin
Catalog No.:BCN5875
CAS No.:107585-75-1
- 4-Demethyl-3,9-dihydroeucomin
Catalog No.:BCN5876
CAS No.:107585-77-3
- 4-Hydroxycoumarin
Catalog No.:BCN2561
CAS No.:1076-38-6
- PF 998425
Catalog No.:BCC7811
CAS No.:1076225-27-8
- Dehydroformouregine
Catalog No.:BCN4054
CAS No.:107633-69-2
- Erycibelline
Catalog No.:BCN1876
CAS No.:107633-95-4
- Merucathinone
Catalog No.:BCN1783
CAS No.:107638-80-2
- Bulleyaconitine A
Catalog No.:BCN1210
CAS No.:107668-79-1
- Merucathine
Catalog No.:BCN1782
CAS No.:107673-74-5
- beta-Lipoic acid
Catalog No.:BCC9198
CAS No.:6992-30-9
- MDL 11,939
Catalog No.:BCC6822
CAS No.:107703-78-6
- Epleremone
Catalog No.:BCC3776
CAS No.:107724-20-9
[Selection of mobile phases for the determination of gamma-schisandrin and multi-active constituents in Schisandra chinensis and its preparations by high performance liquid chromatography].[Pubmed:19803136]
Se Pu. 2009 May;27(3):313-7.
The systems of mobile phase for the determination of gamma-schisandrin in Schisandra chinensis and its preparations were developed by high performance liquid chromatography (HPLC). The separation was performed on a Shim-pack VP-ODS column (250 mm x 4.6 mm, 5 microm) at 30 degrees C, the detection wavelength was set at 285 nm and the flow rate was 1.0 mL/min. Retention times and separation were investigated in mixed solution of three reference substances (gamma-schisandrin, Anwulignan, and deoxyschizandrin) and methanol extract of Schisandra chinensis by different systems and proportions of mobile phases to select optimal conditions for the determination of gamma-schisandrin. The results showed that the complete separation of gamma-schisandrin and Anwulignan was difficult in the systems of methanol-water and methanol-acetic acid-water. The separation of gamma-schisandrin, Anwulignan and deoxyschizandrin can be completed in the systems of acetonitrile-methanol-water and acetonitrile-acetic acid-water when their proportions were suitable. The mobile phase of acetonitrile-methanol-water (17:58:25, v/v/v) was selected for the determination of deoxyschizandrin, gamma-schisandrin and Anwulignan in Schisandra chinensis and Hugan tablets. The determination results of the three substances were satisfactory with the relative standard deviations (n = 4) ranged from 0.95% to 5.8%, and the average recoveries ranged from 94.50% to 105.6%. The efficiency of separation and the results of determination were satisfactory for the real samples.


