MDL 11,9395-HT2A antagonist CAS# 107703-78-6 |
- Necrosulfonamide
Catalog No.:BCC7992
CAS No.:1360614-48-7
- DAPK Substrate Peptide
Catalog No.:BCC2400
CAS No.:386769-53-5
- Cesium chloride
Catalog No.:BCC2399
CAS No.:7647-17-8
- TLQP 21
Catalog No.:BCC2405
CAS No.:869988-94-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 107703-78-6 | SDF | Download SDF |
| PubChem ID | 71781 | Appearance | Powder |
| Formula | C20H25NO | M.Wt | 295.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in ethanol and to 50 mM in DMSO | ||
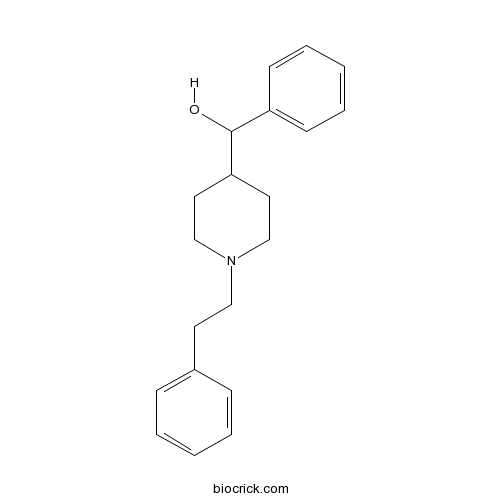
| Chemical Name | phenyl-[1-(2-phenylethyl)piperidin-4-yl]methanol | ||
| SMILES | C1CN(CCC1C(C2=CC=CC=C2)O)CCC3=CC=CC=C3 | ||
| Standard InChIKey | AXNGJCOYCMDPQG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H25NO/c22-20(18-9-5-2-6-10-18)19-12-15-21(16-13-19)14-11-17-7-3-1-4-8-17/h1-10,19-20,22H,11-16H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Orally active 5-HT2A receptor antagonist; displays selectivity for 5-HT2A receptors over 5-HT2C receptors (Ki values are 0.54, 2.5, 81.6 and ~10,000 nM at rabbit 5-HT2A, human 5-HT2A, rabbit 5-HT2C and human 5-HT2C receptors respectively). |

MDL 11,939 Dilution Calculator

MDL 11,939 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.385 mL | 16.9251 mL | 33.8501 mL | 67.7002 mL | 84.6253 mL |
| 5 mM | 0.677 mL | 3.385 mL | 6.77 mL | 13.54 mL | 16.9251 mL |
| 10 mM | 0.3385 mL | 1.6925 mL | 3.385 mL | 6.77 mL | 8.4625 mL |
| 50 mM | 0.0677 mL | 0.3385 mL | 0.677 mL | 1.354 mL | 1.6925 mL |
| 100 mM | 0.0339 mL | 0.1693 mL | 0.3385 mL | 0.677 mL | 0.8463 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- beta-Lipoic acid
Catalog No.:BCC9198
CAS No.:6992-30-9
- Merucathine
Catalog No.:BCN1782
CAS No.:107673-74-5
- Bulleyaconitine A
Catalog No.:BCN1210
CAS No.:107668-79-1
- Merucathinone
Catalog No.:BCN1783
CAS No.:107638-80-2
- Erycibelline
Catalog No.:BCN1876
CAS No.:107633-95-4
- Dehydroformouregine
Catalog No.:BCN4054
CAS No.:107633-69-2
- PF 998425
Catalog No.:BCC7811
CAS No.:1076225-27-8
- 4-Hydroxycoumarin
Catalog No.:BCN2561
CAS No.:1076-38-6
- 4-Demethyl-3,9-dihydroeucomin
Catalog No.:BCN5876
CAS No.:107585-77-3
- 3'-Hydroxy-3,9-dihydroeucomin
Catalog No.:BCN5875
CAS No.:107585-75-1
- Anwulignan
Catalog No.:BCN5362
CAS No.:107534-93-0
- Omadacycline tosylate
Catalog No.:BCC5136
CAS No.:1075240-43-5
- Epleremone
Catalog No.:BCC3776
CAS No.:107724-20-9
- Methyl 7beta,15-dihydroxydehydroabietate
Catalog No.:BCN7270
CAS No.:107752-10-3
- Zafirlukast
Catalog No.:BCC4881
CAS No.:107753-78-6
- A 61603 hydrobromide
Catalog No.:BCC6912
CAS No.:107756-30-9
- Coccinic acid
Catalog No.:BCN5877
CAS No.:107783-45-9
- 3,4-Dihydroxybenzenepropanoic acid
Catalog No.:BCN8500
CAS No.:1078-61-1
- PF 04418948
Catalog No.:BCC6299
CAS No.:1078166-57-0
- Exemestane
Catalog No.:BCC1061
CAS No.:107868-30-4
- Quinovin
Catalog No.:BCN5878
CAS No.:107870-05-3
- Dehydrodiconiferyl alcohol 4-O-beta-D-glucopyranoside
Catalog No.:BCN7707
CAS No.:107870-88-2
- Japonicones D
Catalog No.:BCN3614
CAS No.:1078711-42-8
- Isoeleutherin
Catalog No.:BCN8315
CAS No.:1078723-14-4
Efficacy trial of the 5-HT2 antagonist MDL 11,939 in patients with generalized anxiety disorder.[Pubmed:7714223]
J Clin Psychopharmacol. 1995 Feb;15(1):20-2.
The purpose of this study was to assess the anxiolytic effect of MDL 11,939, a selective 5-HT2 receptor antagonist, in patients with generalized anxiety disorder. After a 1-week placebo lead-in period, 72 healthy male outpatients meeting DSM-III-R criteria for generalized anxiety disorder were randomized to MDL 11,939, 32 mg thrice daily (N = 37), or placebo (N = 35) for 6 weeks. At the end of treatment, MDL 11,939 showed a 7.2-point (30%) decrease in Hamilton Rating Scale for Anxiety scores compared with a 5.7-point (23%) decrease with placebo, but the difference was not significant (p > 0.05). The incidence of adverse events between treatments was similar. MDL 11,939 was well tolerated but did not demonstrate significant anxiolytic effects in this pilot study.
5-HT2A receptor antagonism by MDL 11,939 during inescapable stress prevents subsequent exaggeration of acoustic startle response and reduced body weight in rats.[Pubmed:19889890]
J Psychopharmacol. 2011 Feb;25(2):289-97.
Activation of central 5-HT(2A) receptor signaling and its subsequent alterations have been implicated in the pathophysiological response to stress and the pathogenesis of stress-associated psychiatric disorders. To further examine the association between alterations in central 5-HT(2A) receptor signaling and the occurrence of stress-induced psychiatric symptoms, the present study, utilizing a learned helplessness stress model in rats, determined whether 5-HT(2A) receptor signaling blockade during stress could prevent the occurrence of stress-induced physical and behavioral abnormalities. Rats subjected to restraint/tail shock for three days developed long-lasting elevated acoustic startle response (ASR) and reduced body weight, compared to non-stressed control animals. However, administration of the selective 5-HT(2A) receptor antagonist, MDL 11,939 (alpha-phenyl-1-(2-phenylethyl)-4-piperidinemethanol), 30 min prior to exposure of the animals to the stress protocol prevented the subsequent occurrence of elevated ASR and reduced body weight in a dose-dependent manner in stressed subjects. Administration of MDL 11,939 to the animals immediately after exposure to the stress protocol also prevented the occurrence of exaggerated ASR, but was not able to normalize body weight. These findings suggest a critical role of the central 5-HT(2A) receptor activation in developing the pathophysiology associated with elevated ASR and reduced body weight during stress. The differential effects of MDL 11,939 on startle response and body weight and its potential clinical significance are discussed.
Effects of MDL 11,939 on action potential and contractile force in cardiac tissues: a comparison with bretylium, clofilium, and sotalol.[Pubmed:1704984]
J Cardiovasc Pharmacol. 1990 Dec;16(6):917-23.
The effect of MDL 11,939 hydrochloride [alpha-phenyl-1-(2-phenylethyl)-4-piperidinemethanol HCl], a novel antiarrhythmic agent, was studied using microelectrode recording techniques. MDL 11,939 (10(-7) - 10(-6) M increased the action potential (AP) duration in both guinea pig papillary muscle and dog Purkinje fiber with a maximum effective concentration of 10(-6) to 3 x 10(-6) M. MDL 11,939 up to and including 10(-6) M did not alter upstroke velocity (Vmax) of the AP in either tissue but 10(-5) M decreased the Vmax in Purkinje fibers. Compared to the other class III antiarrhythmic agents (bretylium, clofilium, and d-sotalol), MDL 11,939 was among the most potent in increasing AP duration in the papillary muscle, while being relatively less effective in increasing AP duration in Purkinje fiber. Bretylium did not increase the AP duration in papillary muscle unless it was reserpinized. MDL 11,939, clofilium, and d-sotalol all produced negative inotropic effects in papillary muscle, whereas bretylium produced a positive inotropic effect. The positive inotropic effect of bretylium was abolished by reserpinization. In papillary muscle preparations depolarized with 22 mM K+, MDL 11,939 at concentrations greater than or equal to 10(-5) M depressed Vmax of the slow AP, suggesting that it inhibited the inward Ca2+ current, and such an effect may contribute to the negative inotropic effect of this agent. In the depolarized preparations, the slow AP duration was still increased by concentrations of MDL 11,939 greater than or equal to 10(-6) M. In conclusion, MDL 11,939 has cellular electrophysiological properties that suggest a class III antiarrhythmic activity.
Antiarrhythmic and electrophysiologic effects of MDL 11,939, a novel class III antiarrhythmic agent in anesthetized dogs.[Pubmed:1700208]
J Cardiovasc Pharmacol. 1990 Sep;16(3):383-93.
MDL 11,939 (alpha-phenyl-1-[2-phenylethyl]-4-piperidine-methanol) is a new class III antiarrhythmic agent that was evaluated for antiarrhythmic activity in anesthetized dogs. Intravenous (i.v.) administration of MDL 11,939 (1, 3, and 10 mg/kg) increased left ventricular effective refractory periods. Q-T interval, and Q-Tc in a dose-related way. The effects of MDL 11,939 on ventricular refractoriness were similar to those observed with administration of identical doses of d-sotalol, with the exception that those produced by MDL 11,939 lasted longer. Intraduodenal administration of 10 mg/kg MDL 11,939 also increased left ventricular effective refractory period (LV ERP). The increase in left ventricular refractoriness produced by MDL 11,939 occurred without a significant increase in QRS duration. MDL 11,939 (10 mg/kg i.v.) also protected against induction of ventricular tachycardia (VT) and ventricular fibrillation (VF) induced with programmed electrical stimulation (PES) in anesthetized dogs with chronic 4- to 7-day myocardial infarctions. In comparison, antiarrhythmic effects of bretylium (10 mg/kg i.v.) against PES-induced ventricular arrhythmias were dependent on additional administration of propranolol (0.1 mg/kg i.v.), whereas propranolol alone (0.1 mg/kg i.v.) was ineffective. The results observed with MDL 11,939 are consistent with its in vitro class III antiarrhythmic action and suggest utility for this agent in treatment of VT and VF.
Design, synthesis, and pharmacological characterization of N- and O-substituted 5,6,7,8-tetrahydro-4H-isoxazolo[4,5-d]azepin-3-ol analogues: novel 5-HT(2A)/5-HT(2C) receptor agonists with pro-cognitive properties.[Pubmed:23301527]
J Med Chem. 2013 Feb 14;56(3):1211-27.
The isoxazol-3-one tautomer of the bicyclic isoxazole, 5,6,7,8-tetrahydro-4H-isoxazolo[4,5-d]azepin-3-ol (THAZ), has previously been shown to be a weak GABA(A) and glycine receptor antagonist. In the present study, the potential in this scaffold has been explored through the synthesis and pharmacological characterization of a series of N- and O-substituted THAZ analogues. The analogues N-Bn-THAZ (3d) and O-Bn-THAZ (4d) were found to be potent agonists of the human 5-HT(2A) and 5-HT(2C) receptors. Judging from an elaborate pharmacological profiling at numerous other CNS targets, the 3d analogue appears to be selective for the two receptors. Administration of 3d substantially improved the cognitive performance of mice in a place recognition Y-maze model, an effect fully reversible by coadministration of the selective 5-HT(2C) antagonist SB242084. In conclusion, as novel bioavailable cognitive enhancers that most likely mediate their effects through 5-HT(2A) and/or 5-HT(2C) receptors, the isoxazoles 3d and 4d constitute interesting leads for further medicinal chemistry development.
Antagonist binding at 5-HT(2A) and 5-HT(2C) receptors in the rabbit: high correlation with the profile for the human receptors.[Pubmed:11020478]
Eur J Pharmacol. 2000 Oct 13;406(2):163-9.
This study examined the binding of serotonin receptor antagonists at the 5-HT(2A) and 5-HT(2C) receptors of the rabbit's cerebral cortex. The 5-HT(2A) receptor was characterized by the binding of [3H]MDL 100,907 (R(+)-alpha-(2, 3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine-methan ol) to cortical membranes and the 5-HT(2C) receptor by the binding of [3H]mesulergine in the presence of the selective 5-HT(2A) receptor ligand spiperone. Both [3H]MDL 100,907 and [3H]mesulergine demonstrated high affinity binding to single sites in rabbit membranes. Based on Scatchard plots of [3H]MDL 100,907 binding, the mean B(max) was 8.5+/-0.7 fmol/mg tissue and the mean K(d) was 33. 1+/-3.5 pM. For [3H]mesulergine binding the mean B(max) was 3.70+/-0. 58 fmol/mg tissue and the mean K(d) was 0.35+/-0.05 nM. Binding of [3H]MDL 100,907 to the 5-HT(2A) receptor and of [3H]mesulergine to the 5-HT(2C) receptor was confirmed by displacement studies with highly selective 5-HT(2A) and 5-HT(2C) receptor ligands. The pharmacological profile of these ligands in rabbits correlated highly with published values for 5-HT(2A) (r=0.91, P<0.001) and 5-HT(2C) (r=0.94, P<0.001) receptors in humans. There was also a high correlation between the profiles for human and rat 5-HT(2C) receptor (r=0.92, P<0.001), but not for 5-HT(2A) receptors (r=0.53, P>0.10). It was concluded that the rabbit provides an appropriate animal model for studies attempting to predict the pharmacology of human 5-HT(2A) and 5-HT(2C) receptors.


