ExemestaneSteroidal aromatase inhibitor,selective and irreversible CAS# 107868-30-4 |
- Letrozole
Catalog No.:BCC1063
CAS No.:112809-51-5
- Anastrozole
Catalog No.:BCC4370
CAS No.:120511-73-1
- Aminoglutethimide
Catalog No.:BCC4368
CAS No.:125-84-8
- Formestane
Catalog No.:BCC4369
CAS No.:566-48-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 107868-30-4 | SDF | Download SDF |
| PubChem ID | 60198 | Appearance | Powder |
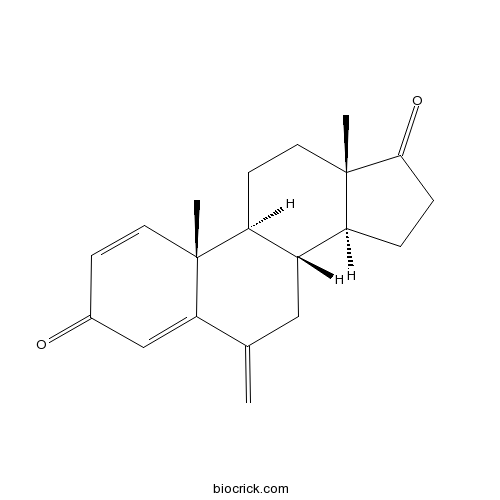
| Formula | C20H24O2 | M.Wt | 296.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | FCE 24304, EXE | ||
| Solubility | DMSO : ≥ 54 mg/mL (182.19 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (8R,9S,10R,13S,14S)-10,13-dimethyl-6-methylidene-7,8,9,11,12,14,15,16-octahydrocyclopenta[a]phenanthrene-3,17-dione | ||
| SMILES | CC12CCC3C(C1CCC2=O)CC(=C)C4=CC(=O)C=CC34C | ||
| Standard InChIKey | BFYIZQONLCFLEV-DAELLWKTSA-N | ||
| Standard InChI | InChI=1S/C20H24O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h6,8,11,14-16H,1,4-5,7,9-10H2,2-3H3/t14-,15-,16-,19+,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Exemestane(FCE 24304) is an aromatase inhibitor, inhibits human placental and rat ovarian aromatase with IC50 of 30 nM and 40 nM, respectively. Target: Aromatase Approved: October 2005 Exemestane competitively inhibits and time-dependently inactivates of human placental aromatase with Ki of 4.3 nM. Exemestane displaces [3H]DHT from rat prostate androgen receptor with IC50 of 0.9 μM . Exemestane (1 μM) increases alkaline phosphatase activity in hFOB and Saos-2 cells and induces the expression of MYBL2, OSTM1, HOXD11, ADCYAP1R1, and glypican 2 in hFOB cells . Exemestane causes aromatase degradation in a dose-responsive manner in MCF-7aro cells . Exemestane increases lumbar spine BMD by 14.0% in OVX rats at dose of 100 mg/kg. Exemestane (100 mg/kg) and 17-hydroexemestane (20 mg/kg) significantly reduces an ovariectomy-induced increase in serum pyridinoline and serum osteocalcin in rats and causes significant reductions of serum cholesterol and low-density lipoprotein cholesterol inOVX rats .Exemestane (20 mg/kg/day s.c.) induces 26% complete (CR) and 18% partial (PR) tumor regressions in rats with 7,12-dimethylbenzanthracene (DMBA)-induced mammary tumors. | |||||

Exemestane Dilution Calculator

Exemestane Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3738 mL | 16.8691 mL | 33.7382 mL | 67.4764 mL | 84.3455 mL |
| 5 mM | 0.6748 mL | 3.3738 mL | 6.7476 mL | 13.4953 mL | 16.8691 mL |
| 10 mM | 0.3374 mL | 1.6869 mL | 3.3738 mL | 6.7476 mL | 8.4345 mL |
| 50 mM | 0.0675 mL | 0.3374 mL | 0.6748 mL | 1.3495 mL | 1.6869 mL |
| 100 mM | 0.0337 mL | 0.1687 mL | 0.3374 mL | 0.6748 mL | 0.8435 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Exemestane is a novel selective and irreversible aromatase inhibitor with an IC50 value of 27 nM. The human placental aromatase was proved to be inhibited by exemestane with Ki of 26 nM and t1/2 of 13.9 min.[1]
Aromatase is a cytochrome P450 enzyme, catalyzing the transformation from androgens to estrogen.[2] Structurally similar to androstenedione, exemestane might have a big impact on androgenic effect. Exemestane can irreversibly inactivate aromatase by interacting with the substrate binding site on the peptide moiety of the enzyme. After binding to the active site, it will be converted to an intermediate that covalently binds to the aromatase binding site, rendering the enzyme inactive. Exemestane has been proved to inhibit aromatase activity in human placental microsomes in-vitro, besides, it can inhibit aromatase activity in the cultured tissue fibroblasts and breast cancer specimens as well. On the other hand, exemestane has been suggested to affect the blood levels and urinary estrogens in-vivo.[3]
References:
[1] Franco Buzzetti, Enrico Di Salle, Antonio Longo, Gabriella Briatico. Synthesis and aromatase inhibition by potential metabolites of exemestane (6-methylenandrosta-1,4-diene-3,17-dione). Steroids. November 1993. 58(11): 527-532.
[2] Gustavo de Albuquerque Cavalcanti, Bruno Carius Garrido, Felipe Dias Leal, Monica Costa Padilha, Xavier de la Torre, Francisco Radler de Aquino Neto. Detection of new urinary exemestane metabolites by gas chromatography coupled to mass spectrometry. Steroids. September–October 2011. 76(10-11): 1010-1015.
[3] Stephanie A. Jones, Stephen E. Jones. Exemestane: A Novel Aromatase Inactivator for Breast Cancer. Clinical Breast Cancer. October 2000. 1(3): 211-216.
- PF 04418948
Catalog No.:BCC6299
CAS No.:1078166-57-0
- 3,4-Dihydroxybenzenepropanoic acid
Catalog No.:BCN8500
CAS No.:1078-61-1
- Coccinic acid
Catalog No.:BCN5877
CAS No.:107783-45-9
- A 61603 hydrobromide
Catalog No.:BCC6912
CAS No.:107756-30-9
- Zafirlukast
Catalog No.:BCC4881
CAS No.:107753-78-6
- Methyl 7beta,15-dihydroxydehydroabietate
Catalog No.:BCN7270
CAS No.:107752-10-3
- Epleremone
Catalog No.:BCC3776
CAS No.:107724-20-9
- MDL 11,939
Catalog No.:BCC6822
CAS No.:107703-78-6
- beta-Lipoic acid
Catalog No.:BCC9198
CAS No.:6992-30-9
- Merucathine
Catalog No.:BCN1782
CAS No.:107673-74-5
- Bulleyaconitine A
Catalog No.:BCN1210
CAS No.:107668-79-1
- Merucathinone
Catalog No.:BCN1783
CAS No.:107638-80-2
- Quinovin
Catalog No.:BCN5878
CAS No.:107870-05-3
- Dehydrodiconiferyl alcohol 4-O-beta-D-glucopyranoside
Catalog No.:BCN7707
CAS No.:107870-88-2
- Japonicones D
Catalog No.:BCN3614
CAS No.:1078711-42-8
- Isoeleutherin
Catalog No.:BCN8315
CAS No.:1078723-14-4
- Ganodermanondiol
Catalog No.:BCN5879
CAS No.:107900-76-5
- Sibiricose A5
Catalog No.:BCN2785
CAS No.:107912-97-0
- 9,9-Bis(4-amino-3-methylphenyl)fluorene
Catalog No.:BCC8794
CAS No.:107934-60-1
- Myricananin A
Catalog No.:BCN5880
CAS No.:1079941-35-7
- 2-(Dimethylamino)ethanol
Catalog No.:BCN1798
CAS No.:108-01-0
- Resorcinol
Catalog No.:BCN5881
CAS No.:108-46-3
- 6-Methyl-5,6-dihydropyran-2-one
Catalog No.:BCN3498
CAS No.:108-54-3
- Melamine
Catalog No.:BCN7248
CAS No.:108-78-1
A phase II study of combined ridaforolimus and dalotuzumab compared with exemestane in patients with estrogen receptor-positive breast cancer.[Pubmed:28324268]
Breast Cancer Res Treat. 2017 Jun;163(3):535-544.
PURPOSE: Combining the mTOR inhibitor ridaforolimus and the anti-IGFR antibody dalotuzumab demonstrated antitumor activity, including partial responses, in estrogen receptor (ER)-positive advanced breast cancer, especially in high proliferation tumors (Ki67 > 15%). METHODS: This randomized, multicenter, international, phase II study enrolled postmenopausal women with advanced ER-positive breast cancer previously treated with a nonsteroidal aromatase inhibitor (NCT01234857). Patients were randomized to either oral ridaforolimus 30 mg daily for 5 of 7 days (once daily [qd] x 5 days/week) plus intravenous dalotuzumab 10 mg/kg/week or oral Exemestane 25 mg/day, and stratified by Ki67 status. Due to a high incidence of stomatitis in the ridaforolimus-dalotuzumab group, two sequential, nonrandomized, reduced-dose cohorts were explored with ridaforolimus 20 and 10 mg qd x 5 days/week. The primary endpoint was progression-free survival (PFS). RESULTS: Median PFS was 21.4 weeks for ridaforolimus 30 mg qd x 5 days/week plus dalotuzumab 10 mg/kg (n = 29) and 24.3 weeks for Exemestane (n = 33; hazard ratio = 1.00; P = 0.5). Overall survival and objective response rates were similar between treatment arms. The incidence of drug-related, nonserious, and serious adverse events was higher with ridaforolimus/dalotuzumab (any ridaforolimus dose) than with Exemestane. Lowering the ridaforolimus dose reduced the incidence of grade 3 stomatitis, but overall toxicity remained higher than acceptable at all doses without improved efficacy. CONCLUSIONS: The combination of ridaforolimus plus dalotuzumab was no more effective than Exemestane in patients with advanced ER-positive breast cancer, and the incidence of adverse events was higher. Therefore, the combination is not being further pursued.
[A Case of Recurrent Breast Cancer with Bone Metastasis Successfully Treated with Everolimus and Exemestane Therapy].[Pubmed:28223674]
Gan To Kagaku Ryoho. 2017 Feb;44(2):157-160.
We report a case of a patient treated with everolimus and Exemestane combination therapy for bone metastasis after breast surgery.The patient, a 58-year-old woman, consulted our department for back pain in October 2014.S he was diagnosed with left breast cancer when she was 41 years old.She had received Bt+Ax for left breast cancer and administered tamoxifen for 5 years.We decided on everolimus and Exemestane combination therapy after observing an abnormal uptake in the 7th to 8th thoracic vertebrae on a PET-CT scan.The pain was controlled using oxycodone and fentanyl orally disintegrating tablet with zoledronic acid.After receiving treatment, the patient experienced pruritus and a Grade 2 rash, but they were managed with antihistamine administration and the treatment was continued.Four months later, the abnormal uptake on the right thoracic vertebrae shrunk; the pain almost disappeared, and oxycodone and fentanyl orally disintegrating tablet were discontinued.Subsequently, Exemestane was used alone.Six months later, the range of abnormal uptake on the thoracic vertebrae progressed, and the disease was evaluated as PD.Four months later, everolimus and Exemestane combination therapy was resumed, and the abnormal uptake on the thoracic vertebrae almost disappeared as observed on a PET scan.The effectiveness of the treatment was evaluated as CR because other local recurrence and new metastases were not found. Everolimus might exhibit bone resorption inhibiting effects and bone protection effects, but the decision regarding the periods of suitable use and the effects of long-term continuation of treatment are controversial, and further discussion based on experience of increasing use is required.
Dose intensity and efficacy of the combination of everolimus and exemestane (EVE/EXE) in a real-world population of hormone receptor-positive (ER+/PgR+), HER2-negative advanced breast cancer (ABC) patients: a multicenter Italian experience.[Pubmed:28353061]
Breast Cancer Res Treat. 2017 Jun;163(3):587-594.
AIM: This retrospective analysis focused on the effect of treatment with EVE/EXE in a real-world population outside of clinical trials. We examined the efficacy of this combination in terms of PFS and RR related to dose intensity (5 mg daily versus 10 mg daily) and tolerability. METHODS: 163 HER2-negative ER+/PgR+ ABC patients, treated with EVE/EXE from May 2011 to March 2016, were included in the analysis. The primary endpoints were the correlation between the daily dose and RR and PFS, as well as an evaluation of the tolerability of the combination. Secondary endpoints were RR, PFS, and OS according to the line of treatment. Patients were classified into three different groups, each with a different dose intensity of everolimus (A, B, C). RESULTS: RR was 29.8% (A), 27.8% (B) (p = 0.953), and not evaluable (C). PFS was 9 months (95% CI 7-11) (A), 10 months (95% CI 9-11) (B), and 5 months (95% CI 2-8) (C), p = 0.956. OS was 38 months (95% CI 24-38) (A), median not reached (B), and 13 months (95% CI 10-25) (C), p = 0.002. Adverse events were stomatitis 57.7% (11.0% grade 3-4), asthenia 46.0% (6.1% grade 3-4), hypercholesterolemia 46.0% (0.6% grade 3-4), and hyperglycemia 35.6% (5.5% grade 3-4). The main reason for discontinuation/interruption was grade 2-3 stomatitis. CONCLUSIONS: No correlation was found between dose intensity (5 vs. 10 mg labeled dose) and efficacy in terms of RR and PFS. The tolerability of the higher dose was poor in our experience, although this had no impact on efficacy.
Quinone-related hexacyclic by-products in the production process of exemestane.[Pubmed:28167101]
Steroids. 2017 Apr;120:26-31.
Exemestane, a 3rd-generation aromatase inhibitor, is clinically used in the treatment of breast cancer in postmenopausal women. The key step of the industrial synthetic process, i.e., a dehydrogenation to introduce the Delta(1)-unsaturation, is normally performed with quinones such as p-chloranil or DDQ. We observed the formation of two different hexacyclic by-products, depending on the quinone used in the oxidation step. These compounds arise from an initial [4+2] cycloaddition between the precursor 6-methylenandrost-4-ene-3,17-dione and the quinone reagent, followed by a twofold dehydrohalogenation (with p-chloranil) or dehydrogenation (with DDQ). The structures of these unprecedented hexacyclic adducts were determined by a combination of mass spectrometry, NMR techniques and crystallographic analysis.
Aromatase inhibitors: are there differences between steroidal and nonsteroidal aromatase inhibitors and do they matter?[Pubmed:18695261]
Oncologist. 2008 Aug;13(8):829-37.
Aromatase inhibitors (AIs) are approved for use in both early- and advanced-stage breast cancer in postmenopausal women. Although the currently approved "third-generation" AIs all powerfully inhibit estrogen synthesis, they may be subdivided into steroidal and nonsteroidal inhibitors, which interact with the aromatase enzyme differently. Nonsteroidal AIs bind noncovalently and reversibly to the aromatase protein, whereas steroidal AIs may bind covalently and irreversibly to the aromatase enzyme. The steroidal AI Exemestane may exert androgenic effects, but the clinical relevance of this has yet to be determined. Switching between steroidal and nonsteroidal AIs produces modest additional clinical benefits, suggesting partial noncrossresistance between the classes of inhibitor. In these circumstances, the response rates to the second AI have generally been low; additional research is needed regarding the optimal sequence of AIs. To date, clinical studies suggest that combining an estrogen-receptor blocker with a nonsteroidal AI does not improve efficacy, while combination with a steroidal AI has not been evaluated. Results from head-to-head trials comparing steroidal and nonsteroidal AIs will determine whether meaningful clinical differences in efficacy or adverse events exist between the classes of AI. This review summarizes the available evidence regarding known differences and evaluates their potential clinical impact.
Aromatase destabilizer: novel action of exemestane, a food and drug administration-approved aromatase inhibitor.[Pubmed:17079446]
Cancer Res. 2006 Nov 1;66(21):10281-6.
Using Western blot as the major technique, we studied the effects of the three Food and Drug Administration (FDA)-approved aromatase inhibitors (AI) on aromatase protein stability in the aromatase-overexpressing breast cancer cell line MCF-7aro. We have found that Exemestane treatment significantly reduces aromatase protein level. Exemestane induces aromatase degradation in a dose-responsive manner (25-200 nmol/L), and the effect can be seen in as early as 2 hours. Metabolic labeling with S(35)-methionine was used to determine the half-life (t(1/2)) of aromatase protein. In the presence of 200 nmol/L Exemestane, the t(1/2) of aromatase was reduced to 12.5 hours from 28.2 hours in the untreated cells. Furthermore, Exemestane-induced aromatase degradation can be completely blocked by 10 micromol/L MG132, indicating that the degradation is mediated by proteasome. We also examined the effect of Exemestane on aromatase mRNA level using real-time reverse transcription-PCR. No significant changes in mRNA level were detected after 8 hours of treatment with Exemestane (200 nmol/L). This is the first report on the evaluation of three FDA-approved AIs on the stability of the aromatase protein. We have found that Exemestane, different from letrozole and anastrozole, can destabilize the aromatase protein.
The steroidal aromatase inhibitor exemestane prevents bone loss in ovariectomized rats.[Pubmed:15003786]
Bone. 2004 Mar;34(3):384-92.
The irreversible steroidal aromatase inhibitor Exemestane (EXE) is one of three third generation aromatase inhibitors currently prescribed for advanced breast cancer in postmenopausal women. Its principal mechanism of action is to reduce estrogen by inhibiting its synthesis. In addition to its efficacy against breast cancer, its effects on other organs are important, especially when given to women with good-prognosis breast cancer or potentially to healthy women at increased risk of developing breast cancer. The purpose of this study was to evaluate the effects of EXE on bone and lipid metabolism in ovariectomized (OVX) rats. Ten-month-old Sprague-Dawley female rats were sorted into intact controls, intact + EXE, OVX controls, and OVX + EXE groups, and treated by weekly intramuscular injection with vehicle or 100 mg/kg EXE for 16 weeks. The bone mineral density (BMD), mechanical testing, histomorphometry, bone resorption marker-serum pyridinoline (PYD), and bone formation marker-serum osteocalcin (OC) were used to determine the effects of treatment on bone. In addition, total serum cholesterol, triglyceride, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were determined. BMD of the lumbar spine and femur were 11% and 7%, respectively, higher in OVX animals given EXE than in OVX controls (all Ps<0.001). Significant increases in the bending strength and toughness of the femora as well as the compressive strength and elastic modulus of the vertebrae were observed in OVX rats given EXE (all Ps<0.02 vs. OVX controls). Trabecular bone volume (BV) was significantly higher in OVX rats treated with EXE than in OVX controls (P<0.0001). In OVX animals, EXE reduced the OVX-induced increase of serum PYD by 96% (P<0.0001), and the OVX-induced increase of serum OC was completely prevented by treatment with EXE. In OVX animals, EXE resulted in a 28% reduction of serum cholesterol (P<0.0001) and reduced LDL by 64% compared with OVX controls (P<0.0001). The positive results of EXE on bone and lipid metabolism in the OVX rat model merit further investigation of the effects of EXE in postmenopausal women.


