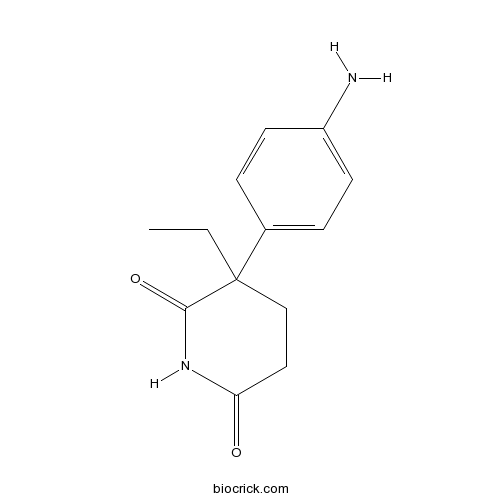
AminoglutethimideCAS# 125-84-8 |
- Nutlin-3a chiral
Catalog No.:BCC1812
CAS No.:675576-98-4
- p53 and MDM2 proteins-interaction-inhibitor chiral
Catalog No.:BCC1830
CAS No.:939981-37-0
- p53 and MDM2 proteins-interaction-inhibitor racemic
Catalog No.:BCC1831
CAS No.:939983-14-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 125-84-8 | SDF | Download SDF |
| PubChem ID | 2145 | Appearance | Powder |
| Formula | C13H16N2O2 | M.Wt | 232.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | DL-Aminoglutethimide | ||
| Solubility | Soluble to 20 mg/mL (86.1 mM) in DMSO | ||
| Chemical Name | 3-(4-aminophenyl)-3-ethylpiperidine-2,6-dione | ||
| SMILES | CCC1(CCC(=O)NC1=O)C2=CC=C(C=C2)N | ||
| Standard InChIKey | ROBVIMPUHSLWNV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Aminoglutethimide is an aromatase inhibitor with IC50 of 10 μM.
Target: Aromatase
Aminoglutethimide inhibits ACTH receptor (ACTH-R) mRNA expression in ovine adrenocortical cells in a time-dependent fashion. Aminoglutethimide significantly suppresses steroid secretion and the baseline ACTH-R mRNA expression in a dose-dependent fashion (300 μM AG, 5±1%; 30 μM AG, 64±1%; 3 μM AG, 108±19% compared with control cells, 100±11%) by affecting the gene expression or by decreasing transcript accumulation via an effect on RNA stability, in the human NCI-h295 adrenocortical carcinoma cell line, which expresses functional ACTH receptors and produces steroids of the glucocorticoid, mineralocorticoid and androgen pathway [1, 2].
Aminoglutethimide (150 mg/kg) abolishes the induction of ornithine decarboxylase (ODC) and almost depletes the gonads and plasma of progesterone or testosterone elicited by human chorionic gonadotropin (hCG) in the ovary of adult female mice and the testis of immature male mice, which is related to an inhibition of cAMP-dependent protein kinase (IC50 287 μM) rather than blockade of the steroidogenic pathway [3]. References: | |||||

Aminoglutethimide Dilution Calculator

Aminoglutethimide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3051 mL | 21.5257 mL | 43.0515 mL | 86.103 mL | 107.6287 mL |
| 5 mM | 0.861 mL | 4.3051 mL | 8.6103 mL | 17.2206 mL | 21.5257 mL |
| 10 mM | 0.4305 mL | 2.1526 mL | 4.3051 mL | 8.6103 mL | 10.7629 mL |
| 50 mM | 0.0861 mL | 0.4305 mL | 0.861 mL | 1.7221 mL | 2.1526 mL |
| 100 mM | 0.0431 mL | 0.2153 mL | 0.4305 mL | 0.861 mL | 1.0763 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Aminoglutethimide is an aromatase inhibitor with IC50 of 10 μM.
- Usnic acid
Catalog No.:BCC8264
CAS No.:125-46-2
- Primidone
Catalog No.:BCC4930
CAS No.:125-33-7
- Vomicine
Catalog No.:BCN6735
CAS No.:125-15-5
- Isobornyl acetate
Catalog No.:BCN8296
CAS No.:125-12-2
- Prednisone 21-acetate
Catalog No.:BCC9128
CAS No.:125-10-0
- Ajugamarin L2
Catalog No.:BCN3662
CAS No.:124961-67-7
- Ajugacumbin A
Catalog No.:BCN3657
CAS No.:124961-66-6
- Raddeanoside R8
Catalog No.:BCN6555
CAS No.:124961-61-1
- Tectoroside
Catalog No.:BCN6672
CAS No.:124960-89-0
- Tolterodine tartrate
Catalog No.:BCC4586
CAS No.:124937-52-6
- (R)-(+)-Tolterodine
Catalog No.:BCC4054
CAS No.:124937-51-5
- Benzoyl leuco methylene blue
Catalog No.:BCC8862
CAS No.:1249-97-4
- 8-Prenylluteone
Catalog No.:BCN4771
CAS No.:125002-91-7
- Fmoc-D-Ala-OPfp
Catalog No.:BCC3037
CAS No.:125043-04-1
- 8-(3-Ethoxy-2-hydroxy-3-methylbutyl)-7-methoxycoumarin
Catalog No.:BCN1594
CAS No.:125072-68-6
- Epinortrachelogenin
Catalog No.:BCN3719
CAS No.:125072-69-7
- XL388
Catalog No.:BCC2059
CAS No.:1251156-08-7
- 26-Nor-8-oxo-alpha-onocerin
Catalog No.:BCN6131
CAS No.:125124-68-7
- 1-(4-methoxyphenyl)-2-methylpropan-1-one
Catalog No.:BCN8163
CAS No.:2040-20-2
- Rosthornin A
Catalog No.:BCN6132
CAS No.:125164-55-8
- Vibralactone D
Catalog No.:BCN6747
CAS No.:1251748-32-9
- N-Methoxyanhydrovobasinediol
Catalog No.:BCN4856
CAS No.:125180-42-9
- Rosthornin B
Catalog No.:BCN6133
CAS No.:125181-21-7
- 1,7-Bis(4-hydroxyphenyl)hept-6-en-3-one
Catalog No.:BCN6597
CAS No.:1251830-57-5
Aminoglutethimide-imprinted xerogels in bulk and spherical formats, based on a multifunctional organo-alkoxysilane precursor.[Pubmed:26589944]
J Chromatogr A. 2015 Dec 11;1424:59-68.
The multifunctional alkoxysilane precursor, 2,6-bis(propyl-trimethoxysilylurelene)pyridine (DPS) was designed and synthesized, envisaging a multiple hydrogen-bond interaction in the molecular imprinting of the drug Aminoglutethimide (AGT). Imprinted xerogels were obtained in bulk and spherical formats. The spherical format was achieved by pore-filling onto spherical mesoporous silica, as a straightforward technique to generate the spherical format. The bulk gels presented better selectivity for the template against its glutarimide (GLU) analogue (selectivity factor: bulk 13.4; spherical 4.6), and good capacity (bulk 5521mumol/L; spherical 2679mumol/L) and imprinting factor parameters (bulk 11.3; spherical 1.4). On the other hand, the microspherical format exhibited better dynamic properties associated to chromatographic efficiency (theoretical plates: bulk 6.8; spherical 75) and mass transfer, due mainly to the existence of a mesoporous network, lacking in the bulk material. The performance of the imprinted xerogels was not as remarkable as that of their acrylic counterparts, previously described. Overall it was demonstrated that the use of designed new "breeds" of organo-alkoxysilanes may be a strategy to achieve satisfactory imprints by the sol-gel processes. DPS may in principle be applied even more effectively to other templates bearing better-matching spatially compatible acceptor-donor-acceptor arrays.
Proteomic profile of aminoglutethimide-induced apoptosis in HL-60 cells: Role of myeloperoxidase and arylamine free radicals.[Pubmed:26102013]
Chem Biol Interact. 2015 Sep 5;239:129-38.
In this study, the cellular effects resulting from the metabolism of Aminoglutethimide by myeloperoxidase were investigated. Human promyelocytic leukemia (HL-60) cells were treated with Aminoglutethimide (AG), an arylamine drug that has a risk of adverse drug reactions, including drug-induced agranulocytosis. HL-60 cells contain abundant amounts of myeloperoxidase (MPO), a hemoprotein, which catalyzes one-electron oxidation of arylamines using H2O2 as a cofactor. Previous studies have shown that arylamine metabolism by MPO results in protein radical formation. The purpose of this study was to determine if pathways associated with a toxic response could be determined from conditions that produced protein radicals. Conditions for AG-induced protein radical formation (with minimal cytotoxicity) were optimized, and these conditions were used to carry out proteomic studies. We identified 43 proteins that were changed significantly upon AG treatment among which 18 were up-regulated and 25 were down-regulated. The quantitative proteomic data showed that AG peroxidative metabolism led to the down-regulation of critical anti-apoptotic proteins responsible for inhibiting the release of pro-apoptotic factors from the mitochondria as well as cytoskeletal proteins such as nuclear lamina. This overall pro-apoptotic response was confirmed with flow cytometry which demonstrated apoptosis to be the main mode of cell death, and this was attenuated by MPO inhibition. This response correlated with the intensity of AG-induced protein radical formation in HL-60 cells, which may play a role in cell death signaling mechanisms.
Enantioseparation of racemic aminoglutethimide using asynchronous simulated moving bed chromatography.[Pubmed:27544751]
J Chromatogr A. 2016 Oct 7;1467:347-355.
The separation of Aminoglutethimide enantiomers by the continuous multicolumn chromatographic processes were investigated experimentally and theoretically, where the columns were packed with cellulose tris 3,5-dimethylphenyl-carbamate stationary phase (brand name Chiralcel OD) and mobile phase was a mixture of n-hexane and ethanol with monoethanolamine additive. The continuous enantioseparation processes included a synchronous shifting process (SMB) and an asynchronous shifting process (VARICOL), which allowed reducing the column number (here from six-column SMB to five-column VARICOL process). Transport-dispersive model with the consideration of both intraparticle mass transfer resistance and axial dispersion was adopted to design and optimize the operation conditions for the separation of Aminoglutethimide enantiomers by SMB process and VARICOL process. According to the optimized operation conditions, experiments were carried out on VARICOL-Micro unit using five-column VARICOL process with 1/1.5/1.5/1 configuration and six-column SMB process with 1/2/2/1 configuration. Products of R-Aminoglutethimide (R-AG) enantiomer and S-Aminoglutethimide (S-AG) enantiomer with more than 99.0% purity were obtained continuously from extract stream and raffinate stream, respectively. Furthermore, the experiemntal data obtained from five-column VARICOL process were compared with that from six-column SMB process, the feasibility and efficiency for the separation of guaifenesin enantiomers by VARICOL processes were evaluated.
[Chromatographic separation of aminoglutethimide enantiomers on cellulose tris(3,5-dimethylphenylcarbamate) chiral stationary phase].[Pubmed:25434126]
Se Pu. 2014 Aug;32(8):880-5.
Aminoglutethimide (AG) has been used clinically as a drug in the treatment of hormone-dependent metastatic breast cancer. It was reported that S-(-)-AG enantiomer had small activity and sometimes might cause side effects. Therefore, it was of great significance to obtain the high-purity R-(+)-AG by enantioseparation. In this work, Aminoglutethimide enantiomers were separated by high performance liquid chromatography (HPLC) using an analytical column which was packed with cellulose tris(3,5-dimethylphenylcarbamate) stationary phase (Chiralcel OD-H). The solubilities of racemic AG in two different solvent compositions, n-hexane/ethanol and n-hexane/isopropanol, were measured, separately. The effects of alcohol content and monoethanolamine additive on the separation performance of racemic AG by HPLC were investigated. According to the experiments, n-hexane-ethanol (30:70, v/v) with 0.1% monoethanolamine additive was selected as the mobile phase. The separation factor, resolution, asymmetry factor, number of theoretical plates and maximum column capacity were measured and analyzed for the chromatographic separation of racemic AG at a flow-rate of 0. 6 mL/min and column temperature of 25-40 degrees C, with Chiralcel OD-H as stationary phase and n-hexane-ethanol (30:70, v/v) with 0. 1% monoethanolamine as mobile phase. This work provides the basic information of chromatographic separation for the batch and continuous production of Aminoglutethimide enantiomers.


