4-HydroxycoumarinCAS# 1076-38-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1076-38-6 | SDF | Download SDF |
| PubChem ID | 15564935 | Appearance | Powder |
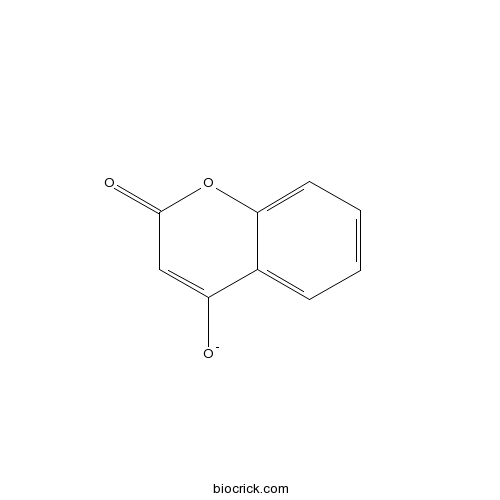
| Formula | C9H6O3 | M.Wt | 162.14 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 250 mg/mL (1541.88 mM; Need ultrasonic) | ||
| Chemical Name | 4-oxochromen-2-olate | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C=C(O2)[O-] | ||
| Standard InChIKey | OWBBAPRUYLEWRR-UHFFFAOYSA-M | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 4-Hydroxycoumarin serves as an immediate precursor of 4-hydroxycoumarin (4HC) type anticoagulants (for example, warfarin). |
| Targets | AChR |
| In vitro | Effect of different C3-aryl substituents on the antioxidant activity of 4-hydroxycoumarin derivatives.[Pubmed: 21964183]Bioorg Med Chem. 2011 Nov 1;19(21):6233-8.The antioxidant activity of 4-Hydroxycoumarin synthetic derivatives and 4-methylumbelliferone were determined taking 4-Hydroxycoumarin as the reference compound. Larvicidal activity of 4-hydroxycoumarin derivatives against Aedes aegypti.[Pubmed: 21043993]Pharm Biol. 2011 Feb;49(2):190-3.During our search for new types of coumarin derivatives possessing a larvicidal activity, we investigated the synthesis of 4-Hydroxycoumarin derivatives. |
| Structure Identification | Chem Biol Drug Des. 2015 May 22.Synthesis and anticholinergic activity of 4-hydroxycoumarin derivatives containing substituted benzyl-1,2,3-triazole moiety.[Pubmed: 26010139]
Nat Commun. 2013;4:2603.Microbial biosynthesis of the anticoagulant precursor 4-hydroxycoumarin.[Pubmed: 24129598]4-Hydroxycoumarin (4HC) type anticoagulants (for example, warfarin) are known to have a significant role in the treatment of thromboembolic diseases--a leading cause of patient morbidity and mortality worldwide. 4HC serves as an immediate precursor of these synthetic anticoagulants. Although 4HC was initially identified as a naturally occurring product, its biosynthesis has not been fully elucidated. |

4-Hydroxycoumarin Dilution Calculator

4-Hydroxycoumarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.1675 mL | 30.8375 mL | 61.6751 mL | 123.3502 mL | 154.1877 mL |
| 5 mM | 1.2335 mL | 6.1675 mL | 12.335 mL | 24.67 mL | 30.8375 mL |
| 10 mM | 0.6168 mL | 3.0838 mL | 6.1675 mL | 12.335 mL | 15.4188 mL |
| 50 mM | 0.1234 mL | 0.6168 mL | 1.2335 mL | 2.467 mL | 3.0838 mL |
| 100 mM | 0.0617 mL | 0.3084 mL | 0.6168 mL | 1.2335 mL | 1.5419 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Demethyl-3,9-dihydroeucomin
Catalog No.:BCN5876
CAS No.:107585-77-3
- 3'-Hydroxy-3,9-dihydroeucomin
Catalog No.:BCN5875
CAS No.:107585-75-1
- Anwulignan
Catalog No.:BCN5362
CAS No.:107534-93-0
- Omadacycline tosylate
Catalog No.:BCC5136
CAS No.:1075240-43-5
- Glucagon-like peptide 1 (7-36) amide (human, rat)
Catalog No.:BCC7258
CAS No.:107444-51-9
- Bisabola-2,10-diene-1,9-dione
Catalog No.:BCN7269
CAS No.:107439-25-8
- Ginkgolide J
Catalog No.:BCN5939
CAS No.:107438-79-9
- CD 1530
Catalog No.:BCC7406
CAS No.:107430-66-0
- Garcinone D
Catalog No.:BCN2526
CAS No.:107390-08-9
- Cleroindicin B
Catalog No.:BCN5874
CAS No.:107389-91-3
- LDC000067
Catalog No.:BCC5452
CAS No.:1073485-20-7
- Demethylzeylasteral
Catalog No.:BCN2282
CAS No.:107316-88-1
- PF 998425
Catalog No.:BCC7811
CAS No.:1076225-27-8
- Dehydroformouregine
Catalog No.:BCN4054
CAS No.:107633-69-2
- Erycibelline
Catalog No.:BCN1876
CAS No.:107633-95-4
- Merucathinone
Catalog No.:BCN1783
CAS No.:107638-80-2
- Bulleyaconitine A
Catalog No.:BCN1210
CAS No.:107668-79-1
- Merucathine
Catalog No.:BCN1782
CAS No.:107673-74-5
- beta-Lipoic acid
Catalog No.:BCC9198
CAS No.:6992-30-9
- MDL 11,939
Catalog No.:BCC6822
CAS No.:107703-78-6
- Epleremone
Catalog No.:BCC3776
CAS No.:107724-20-9
- Methyl 7beta,15-dihydroxydehydroabietate
Catalog No.:BCN7270
CAS No.:107752-10-3
- Zafirlukast
Catalog No.:BCC4881
CAS No.:107753-78-6
- A 61603 hydrobromide
Catalog No.:BCC6912
CAS No.:107756-30-9
Microbial biosynthesis of the anticoagulant precursor 4-hydroxycoumarin.[Pubmed:24129598]
Nat Commun. 2013;4:2603.
4-Hydroxycoumarin (4HC) type anticoagulants (for example, warfarin) are known to have a significant role in the treatment of thromboembolic diseases--a leading cause of patient morbidity and mortality worldwide. 4HC serves as an immediate precursor of these synthetic anticoagulants. Although 4HC was initially identified as a naturally occurring product, its biosynthesis has not been fully elucidated. Here we present the design, validation, in vitro diagnosis and optimization of an artificial biosynthetic mechanism leading to the microbial biosynthesis of 4HC. Remarkably, function-based enzyme bioprospecting leads to the identification of a characteristic FabH-like quinolone synthase from Pseudomonas aeruginosa with high efficiency on the 4HC-forming reaction, which promotes the high-level de novo biosynthesis of 4HC in Escherichia coli (~500 mg l(-)(1) in shake flasks) and further in situ semisynthesis of warfarin. This work has the potential to be scaled-up for microbial production of 4HC and opens up the possibility of biosynthesizing diverse coumarin molecules with pharmaceutical importance.
Larvicidal activity of 4-hydroxycoumarin derivatives against Aedes aegypti.[Pubmed:21043993]
Pharm Biol. 2011 Feb;49(2):190-3.
CONTEXT: Coumarins are natural substances found in a variety of plants. It is well known that plant-derived natural products are extensively used as biologically active compounds. Among them, coumarins were the first preservatives used by man, originally in their natural state within plant tissues and then as natural materials obtained by water distillation. During our search for new types of coumarin derivatives possessing a larvicidal activity, we investigated the synthesis of 4-Hydroxycoumarin derivatives. OBJECTIVE: The coumarin derivatives were synthesized and the structure determination and larvicidal effects were studied. MATERIALS AND METHODS: The structure analyses were conducted by nuclear magnetic resonance (NMR), and mass (MS) spectroscopy revealed that the coumarin derivatives were obtained in good yields, and the eight coumarin derivatives were 3-{1,2,3,4-tetrahydro-3-[4-(4-trifluoromethylbenzyloxy)phenyl}-1-naphthalen-1-on (1), 3-{1,2,3,4-tetrahydro-3-[4-(4-trifluoro methylbenzyloxy)phenyl}-1-naphthalen-1-ol (2), brodifacoum (3), difethialone (4), bromadiolone (5), 4-hydroxy-3-(1,2,3,4-tetrahydronaphthalen-1-yl)-2H-chromen-2-one (coumatetralyl) (6), cis-flocoumafen (7) and trans-flocoumafen (8). RESULTS: The compounds were tested against the F(21) laboratory strain of Aedes aegypti L. Brodifacoum and cis-flocoumafen mediated strong activity with an LC(50) values of 8.23 and 9.34 ppm, respectively. DISCUSSION AND CONCLUSION: The above indicates that brodifacoum may play a more important role in the toxicity of 4-Hydroxycoumarin derivatives.
Synthesis and anticholinergic activity of 4-hydroxycoumarin derivatives containing substituted benzyl-1,2,3-triazole moiety.[Pubmed:26010139]
Chem Biol Drug Des. 2015 Nov;86(5):1215-20.
A series of 4-Hydroxycoumarin-derived compounds 8a-p containing N-benzyl-1,2,3-triazole motif were designed as AChE inhibitors. The title compounds were obtained conveniently using multicomponent click reaction. The in vitro anticholinesterase evaluation of synthesized compounds against AChE and BuChE showed that some of them are potent and selective inhibitors of AChE. Among them, 2-chlorobenzyl derivative 8k showed the most potent activity against AChE (IC50 = 0.18 mum). Its activity was also superior to that of standard drug tacrine. The kinetic study and molecular docking simulation of the most potent compound 8k were also described.
Effect of different C3-aryl substituents on the antioxidant activity of 4-hydroxycoumarin derivatives.[Pubmed:21964183]
Bioorg Med Chem. 2011 Nov 1;19(21):6233-8.
The antioxidant activity of 4-Hydroxycoumarin synthetic derivatives and 4-methylumbelliferone were determined taking 4-Hydroxycoumarin as the reference compound. Six 3-aryl-4-Hydroxycoumarin derivatives were synthesized from 4-Hydroxycoumarin as precursor in order to evaluate changes in their antioxidant properties due to C3-aryl substituent nature. Free radical scavenging capacities of these compounds against two different species DPPH(.) and ABTS(.+) and the protecting ability towards the beta-carotene-linoleic acid co-oxidation enzymatically induced by lipoxygenase were measured. In addition, the relationship between the activities of these molecules against DPPH radical and the bond dissociation energy of O-H (BDE) calculated using methods of computational chemistry was evaluated.


