DefactinibCAS# 1073154-85-4 |
- Brefeldin A
Catalog No.:BCC4387
CAS No.:20350-15-6
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Vitamin D3
Catalog No.:BCN2186
CAS No.:67-97-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1073154-85-4 | SDF | Download SDF |
| PubChem ID | 25117126 | Appearance | Powder |
| Formula | C20H21F3N8O3S | M.Wt | 510.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | VS-6063; PF-04554878 | ||
| Solubility | DMSO : ≥ 39 mg/mL (76.40 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
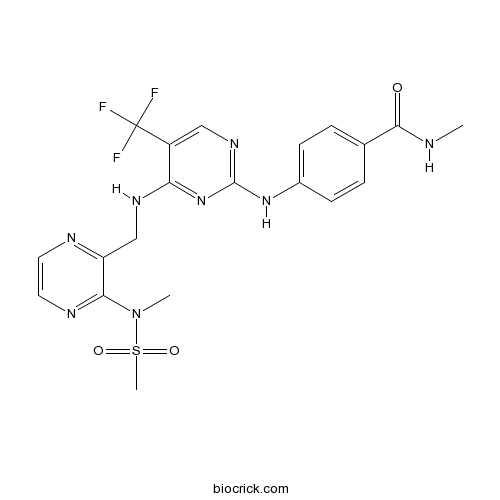
| Chemical Name | N-methyl-4-[[4-[[3-[methyl(methylsulfonyl)amino]pyrazin-2-yl]methylamino]-5-(trifluoromethyl)pyrimidin-2-yl]amino]benzamide | ||
| SMILES | CNC(=O)C1=CC=C(C=C1)NC2=NC=C(C(=N2)NCC3=NC=CN=C3N(C)S(=O)(=O)C)C(F)(F)F | ||
| Standard InChIKey | FWLMVFUGMHIOAA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H21F3N8O3S/c1-24-18(32)12-4-6-13(7-5-12)29-19-28-10-14(20(21,22)23)16(30-19)27-11-15-17(26-9-8-25-15)31(2)35(3,33)34/h4-10H,11H2,1-3H3,(H,24,32)(H2,27,28,29,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Defactinib Dilution Calculator

Defactinib Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9589 mL | 9.7945 mL | 19.589 mL | 39.178 mL | 48.9726 mL |
| 5 mM | 0.3918 mL | 1.9589 mL | 3.9178 mL | 7.8356 mL | 9.7945 mL |
| 10 mM | 0.1959 mL | 0.9795 mL | 1.9589 mL | 3.9178 mL | 4.8973 mL |
| 50 mM | 0.0392 mL | 0.1959 mL | 0.3918 mL | 0.7836 mL | 0.9795 mL |
| 100 mM | 0.0196 mL | 0.0979 mL | 0.1959 mL | 0.3918 mL | 0.4897 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Defactinib is a novel FAK inhibitor, which inhibits FAK phosphorylation at the Tyr397 site in a time- and dose-dependent manner.
In Vitro:VS-6063 inhibits FAK phosphorylation at the Tyr397 site in a time- and dose-dependent manner. The combination of VS-6063 and Paclitaxel markedly decreases proliferation and increases apoptosis, which results in 92.7% to 97.9% reductions in tumor weight. RPPA data shows that VS-6063 reduces levels of AKT and YB-1 in taxane-resistant cell lines. The expression of pFAK (Tyr397) is statistically significantly inhibited by VS-6063 in a dose-dependent manner in all cell lines. VS-6063 inhibits pFAK (Tyr397) expression within 3 hours, with a gradual return of expression by 48 hours[1].
In Vivo:VS-6063 doses of 25 mg/kg twice a day or greater statistically significantly inhibits pFAK (Tyr397) at 3 hours, with return of expression noted by 24 hours. Therefore, administration of VS-6063 at 25 mg/kg twice a day is selected as the dosing schedule for subsequent therapy experiments. For therapy experiments, female nude mice bearing HeyA8 tumors in the peritoneal cavity are randomly divided into 4 groups (n=10 per group): 1) vehicle orally twice daily and phosphate-buffered saline intraperitoneally weekly (control); 2) VS-6063 25 mg/kg orally twice daily; 3) PTX intraperitoneally weekly; and 4) both VS-6063 25 mg/kg orally twice daily and PTX intraperitoneally weekly. There is an 87.4% reduction in tumor weight by PTX monotherapy in the HeyA8 model, and combination therapy resulted in the greatest tumor weight reduction, with a 97.9% reduction (P=0.05 compared with PTX). In the SKOV3ip1 model, a 92.7% tumor weight reduction is observed in the combination group compared with PTX (P<0.001)[1].
References:
[1]. Kang Y, et al. Role of focal adhesion kinase in regulating YB-1-mediated paclitaxel resistance in ovarian cancer. J Natl Cancer Inst. 2013 Oct 2;105(19):1485-95.
- SR-3677
Catalog No.:BCC4302
CAS No.:1072959-67-1
- MLN2238
Catalog No.:BCC2092
CAS No.:1072833-77-2
- Carasinol D
Catalog No.:BCN8228
CAS No.:1072797-66-0
- Baogongteng C
Catalog No.:BCN1873
CAS No.:107259-50-7
- NPPB
Catalog No.:BCC6711
CAS No.:107254-86-4
- 2-[(1S)-2-Formyl-1,3,3-trimethylcyclohexyl]-4-hydroxy-5-propan-2-ylbenzaldehyde
Catalog No.:BCN3584
CAS No.:1072444-55-3
- Cevimeline
Catalog No.:BCC1470
CAS No.:107233-08-9
- Epigoitrin
Catalog No.:BCN6278
CAS No.:1072-93-1
- AT-406 (SM-406)
Catalog No.:BCC1283
CAS No.:1071992-99-8
- Deoxyflindissone
Catalog No.:BCN7268
CAS No.:107176-31-8
- MAC13243
Catalog No.:BCC1727
CAS No.:1071638-38-4
- Pyrocincholic acid methyl ester
Catalog No.:BCN5873
CAS No.:107160-24-7
- Demethylzeylasteral
Catalog No.:BCN2282
CAS No.:107316-88-1
- LDC000067
Catalog No.:BCC5452
CAS No.:1073485-20-7
- Cleroindicin B
Catalog No.:BCN5874
CAS No.:107389-91-3
- Garcinone D
Catalog No.:BCN2526
CAS No.:107390-08-9
- CD 1530
Catalog No.:BCC7406
CAS No.:107430-66-0
- Ginkgolide J
Catalog No.:BCN5939
CAS No.:107438-79-9
- Bisabola-2,10-diene-1,9-dione
Catalog No.:BCN7269
CAS No.:107439-25-8
- Glucagon-like peptide 1 (7-36) amide (human, rat)
Catalog No.:BCC7258
CAS No.:107444-51-9
- Omadacycline tosylate
Catalog No.:BCC5136
CAS No.:1075240-43-5
- Anwulignan
Catalog No.:BCN5362
CAS No.:107534-93-0
- 3'-Hydroxy-3,9-dihydroeucomin
Catalog No.:BCN5875
CAS No.:107585-75-1
- 4-Demethyl-3,9-dihydroeucomin
Catalog No.:BCN5876
CAS No.:107585-77-3
Maintenance Defactinib Versus Placebo After First-Line Chemotherapy in Patients With Merlin-Stratified Pleural Mesothelioma: COMMAND-A Double-Blind, Randomized, Phase II Study.[Pubmed:30785827]
J Clin Oncol. 2019 Apr 1;37(10):790-798.
PURPOSE: Inhibition of focal adhesion kinase has been shown to selectively kill mesothelioma cells that express low levels of moesin-ezrin-radixin-like protein (merlin). On this basis, we designed a randomized, phase II trial to investigate whether Defactinib as maintenance therapy after standard first-line chemotherapy could improve progression-free survival (PFS) in patients with malignant pleural mesothelioma (MPM). METHODS: This global, double-blind, randomized, placebo-controlled trial was conducted in patients with advanced MPM and disease control after at least four cycles of first-line chemotherapy. Patients were stratified for merlin and then randomly assigned (in a 1:1 fashion) to receive either oral Defactinib or placebo until disease progression, unacceptable toxicity, or withdrawal occurred. The coprimary end points were PFS and overall survival (OS). Quality of life (QoL) was assessed using the Lung Cancer Symptom Scale for Mesothelioma tool. RESULTS: Three hundred forty-four patients were randomly assigned to receive either Defactinib (n = 173) or placebo (n = 171). The median PFS was 4.1 months (95% CI, 2.9 to 5.6 months) for Defactinib versus 4.0 months (95% CI, 2.9 to 4.2 months) for placebo. The median OS was 12.7 months (95% CI, 9.1 to 21 months) for Defactinib versus 13.6 months (95% CI, 9.6 to 21.2 months) for placebo (hazard ratio, 1.0; 95% CI, 0.7 to 1.4). Although shorter survival for both Defactinib- and placebo-treated patients was observed, in the patients who had merlin-low MPM compared with the patients who had merlin-high MPM, there were no statistical differences in response rate, PFS, OS, or QoL between the treatment groups. The most common grade 3 or worse adverse events were nausea, diarrhea, fatigue, dyspnea, and decreased appetite. CONCLUSION: Neither PFS nor OS was improved by Defactinib after first-line chemotherapy in patients with merlin-low MPM. Defactinib cannot be recommended as maintenance therapy for advanced MPM.
LC-ESI-MS/MS determination of defactinib, a novel FAK inhibitor in mice plasma and its application to a pharmacokinetic study in mice.[Pubmed:29145097]
J Pharm Biomed Anal. 2018 Feb 5;149:358-364.
A sensitive, specific, selective and rapid LC-ESI-MS/MS method has been developed and validated for the quantification of Defactinib in mice plasma using (13)C3,(15)N-tofacitinib as an internal standard (I.S.). Sample preparation was accomplished through a liquid-liquid extraction process. Baseline chromatographic resolution of Defactinib and the I.S. was achieved on an Atlantis dC18 column using an isocratic mobile phase comprising 0.2% formic acid in water and acetonitrile (25:75, v/v) delivered at a flow rate of 0.5mL/min. Defactinib and the I.S. eluted at approximately 1.59 and 0.99min, respectively. The total chromatographic run time was 2.50min. A linear response function was established in the concentration range of 0.13-106 ng/mL. Method validation was performed as per regulatory guidelines and the results met the acceptance criteria. The intra- and inter-day accuracy and precision were in the range of 5.57-13.3 and 8.63-12.1%, respectively. Defactinib was found to be stable under various stability conditions. This novel method has been applied to a pharmacokinetic study in mice.


