AHU-377(Sacubitril)Neprilysin inhibitor CAS# 149709-62-6 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 149709-62-6 | SDF | Download SDF |
| PubChem ID | 9811834 | Appearance | Powder |
| Formula | C24H29NO5 | M.Wt | 411.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Sacubitril | ||
| Solubility | DMSO : ≥ 100 mg/mL (243.02 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-[[(2S,4R)-5-ethoxy-4-methyl-5-oxo-1-(4-phenylphenyl)pentan-2-yl]amino]-4-oxobutanoic acid | ||
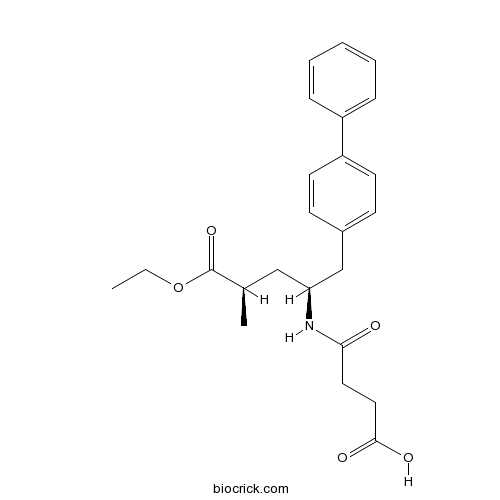
| SMILES | CCOC(=O)C(C)CC(CC1=CC=C(C=C1)C2=CC=CC=C2)NC(=O)CCC(=O)O | ||
| Standard InChIKey | PYNXFZCZUAOOQC-UTKZUKDTSA-N | ||
| Standard InChI | InChI=1S/C24H29NO5/c1-3-30-24(29)17(2)15-21(25-22(26)13-14-23(27)28)16-18-9-11-20(12-10-18)19-7-5-4-6-8-19/h4-12,17,21H,3,13-16H2,1-2H3,(H,25,26)(H,27,28)/t17-,21+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AHU-377 is an inhibitor of neprilysin with IC50 value of 5 nM. | |||||
| Targets | neprilysin | |||||
| IC50 | 5 nM | |||||

AHU-377(Sacubitril) Dilution Calculator

AHU-377(Sacubitril) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4302 mL | 12.151 mL | 24.3019 mL | 48.6039 mL | 60.7548 mL |
| 5 mM | 0.486 mL | 2.4302 mL | 4.8604 mL | 9.7208 mL | 12.151 mL |
| 10 mM | 0.243 mL | 1.2151 mL | 2.4302 mL | 4.8604 mL | 6.0755 mL |
| 50 mM | 0.0486 mL | 0.243 mL | 0.486 mL | 0.9721 mL | 1.2151 mL |
| 100 mM | 0.0243 mL | 0.1215 mL | 0.243 mL | 0.486 mL | 0.6075 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AHU-377 is an inhibitor of neprilysin with IC50 value of 5nM [1].
AHU-377 and the angiotensin II AT1 receptor antagonist valsartan compose LCZ696 in a 1:1 molar ratio. LCZ696 is an angiotensin receptor neprilysin inhibitor. It can reduce blood pressure and may be a novel drug for the treatment of heart failure. AHU-377 is a prodrug, it can be converted by enzymatic cleavage of the ethyl ester into the active form LBQ657. It is reported that AHU-377(30 and 100 mg/kg, PO) can cause antihypertensive effect in a dose-dependent manner in Dahl-SS rats. But in the DOCA-salt hypertensive rats, it shows a weak reduction [2, 3].
References:
[1] Ksander GM, Ghai RD, deJesus R, Diefenbacher CG, Yuan A, Berry C, Sakane Y, Trapani A. Dicarboxylic acid dipeptide neutral endopeptidase inhibitors. J Med Chem. 1995 May 12;38(10):1689-700.
[2] Voors AA, Dorhout B, van der Meer P. The potential role of valsartan + AHU377 ( LCZ696 ) in the treatment of heart failure. Expert Opin Investig Drugs. 2013 Aug;22(8):1041-7.
[3] Laxminarayan G Hegde, Cecile Yu, Cheruvu Madhavi et al. Comparative efficacy of AHU-377, a potent neprilysin inhibitor, in two rat models of volume-dependent hypertension. BMC Pharmacology 2011, 11(Suppl 1):P33.
- Stachybotrolide
Catalog No.:BCN6968
CAS No.:149691-31-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Isomorellinol
Catalog No.:BCN3075
CAS No.:149655-53-8
- Isogambogic acid
Catalog No.:BCN3078
CAS No.:149655-52-7
- Chrysosplenol D
Catalog No.:BCN1666
CAS No.:14965-20-9
- Nafadotride
Catalog No.:BCC7025
CAS No.:149649-22-9
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
- MOG (35-55)
Catalog No.:BCC3670
CAS No.:149635-73-4
- Impentamine dihydrobromide
Catalog No.:BCC7197
CAS No.:149629-70-9
- Stachybotramide
Catalog No.:BCN6969
CAS No.:149598-71-0
- Marmin
Catalog No.:BCN1665
CAS No.:14957-38-1
- Ganoderic acid AM1
Catalog No.:BCN2441
CAS No.:149507-55-1
- RS 23597-190 hydrochloride
Catalog No.:BCC6767
CAS No.:149719-06-2
- H-Arg-NH2.2HCl
Catalog No.:BCC2859
CAS No.:14975-30-5
- Tereticornate A
Catalog No.:BCN1667
CAS No.:149751-81-5
- Clemastine Fumarate
Catalog No.:BCC4528
CAS No.:14976-57-9
- Delaminomycin A
Catalog No.:BCN1833
CAS No.:149779-38-4
- Homoeriodictyol 7-O-glucoside
Catalog No.:BCN7740
CAS No.:14982-11-7
- Saquinavir mesylate
Catalog No.:BCC1922
CAS No.:149845-06-7
- 2-MPMDQ
Catalog No.:BCC6741
CAS No.:149847-77-8
- AAL Toxin TB1
Catalog No.:BCN1734
CAS No.:149849-90-1
- AAL Toxin TB2
Catalog No.:BCN1739
CAS No.:149849-91-2
- Azimilide Dihydrochloride
Catalog No.:BCC5536
CAS No.:149888-94-8
- H-Glu(OMe)-OH
Catalog No.:BCC2931
CAS No.:1499-55-4
Pharmacokinetic, pharmacodynamic, and antihypertensive effects of the neprilysin inhibitor LCZ-696: sacubitril/valsartan.[Pubmed:28652105]
J Am Soc Hypertens. 2017 Jul;11(7):461-468.
LCZ-696, sacubitril/valsartan, is a dual-acting molecule consisting of the angiotensin II (Ang II) receptor blocker valsartan and the neprilysin (neutral endopeptidase) inhibitor AHU-377 with significant beneficial effects in patients with hypertension and heart failure (HF). Several recent studies have demonstrated a higher effectiveness of LCZ-696 compared to valsartan in the treatment of hypertension and HF. The rationale for the development and the Food and Drug Administration approval of LCZ-696 was based on the concept of an additive effect of the Ang II receptor blocker valsartan and the neutral endopeptidase (neprilysin) inhibitor AHU-377 for the treatment of hypertension and HF. The synergism from these drugs arises from the vasodilating effects of valsartan through its blockade of Ang II type 1 receptor and the action of natriuretic peptides atrial natriuretic peptide and B-type natriuretic peptide (BNP) by preventing their catabolism with neprilysin resulting in increase of cyclic guanosine monophosphate. This action of neprilysin is associated with increased natriuresis, diuresis, and systemic vasodilation, since these peptides have been shown to have potent diuretic, natriuretic, and vasodilating effects. In addition, it reduces the levels of N terminal pro-BNP. Therefore, administration of LCZ-696 results in significant reduction of wall stress from pressure and volume overload of the left ventricle as demonstrated by the reduction of N terminal pro-BNP, both significant constituents of hypertension and HF, and it is safe, well tolerated and is almost free of cough and angioedema.


