IsomorellinolCAS# 149655-53-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 149655-53-8 | SDF | Download SDF |
| PubChem ID | 70639868 | Appearance | Powder |
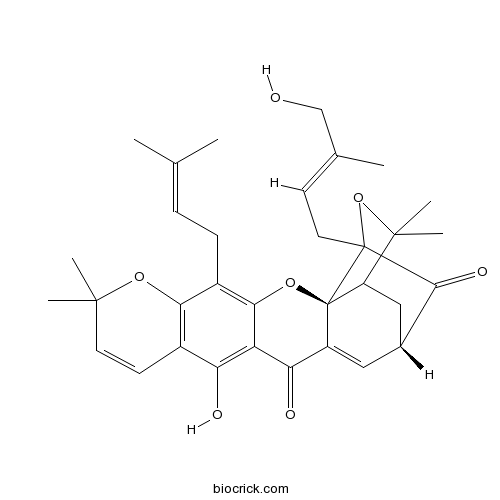
| Formula | C33H38O7 | M.Wt | 546.7 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC(=CCC1=C2C(=C(C3=C1OC(C=C3)(C)C)O)C(=O)C4=CC5CC6C4(O2)C(C5=O)(OC6(C)C)CC=C(C)CO)C | ||
| Standard InChIKey | UWZMGTSPGQXAAP-FRMWRBSQSA-N | ||
| Standard InChI | InChI=1S/C33H38O7/c1-17(2)8-9-21-27-20(11-12-30(4,5)38-27)25(35)24-26(36)22-14-19-15-23-31(6,7)40-32(29(19)37,13-10-18(3)16-34)33(22,23)39-28(21)24/h8,10-12,14,19,23,34-35H,9,13,15-16H2,1-7H3/b18-10+/t19-,23?,32?,33-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Isomorellinol induces apoptosis in cholangiocarcinoma (CCA)cells which is mediated through a mitochondria-dependent signaling pathway. |

Isomorellinol Dilution Calculator

Isomorellinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8292 mL | 9.1458 mL | 18.2916 mL | 36.5831 mL | 45.7289 mL |
| 5 mM | 0.3658 mL | 1.8292 mL | 3.6583 mL | 7.3166 mL | 9.1458 mL |
| 10 mM | 0.1829 mL | 0.9146 mL | 1.8292 mL | 3.6583 mL | 4.5729 mL |
| 50 mM | 0.0366 mL | 0.1829 mL | 0.3658 mL | 0.7317 mL | 0.9146 mL |
| 100 mM | 0.0183 mL | 0.0915 mL | 0.1829 mL | 0.3658 mL | 0.4573 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isogambogic acid
Catalog No.:BCN3078
CAS No.:149655-52-7
- Chrysosplenol D
Catalog No.:BCN1666
CAS No.:14965-20-9
- Nafadotride
Catalog No.:BCC7025
CAS No.:149649-22-9
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
- MOG (35-55)
Catalog No.:BCC3670
CAS No.:149635-73-4
- Impentamine dihydrobromide
Catalog No.:BCC7197
CAS No.:149629-70-9
- Stachybotramide
Catalog No.:BCN6969
CAS No.:149598-71-0
- Marmin
Catalog No.:BCN1665
CAS No.:14957-38-1
- Ganoderic acid AM1
Catalog No.:BCN2441
CAS No.:149507-55-1
- Hypocrellin C
Catalog No.:BCN3398
CAS No.:149457-83-0
- Traxillaside
Catalog No.:BCN6917
CAS No.:149415-62-3
- Poncirin
Catalog No.:BCN2590
CAS No.:14941-08-3
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Stachybotrolide
Catalog No.:BCN6968
CAS No.:149691-31-6
- AHU-377(Sacubitril)
Catalog No.:BCC4088
CAS No.:149709-62-6
- RS 23597-190 hydrochloride
Catalog No.:BCC6767
CAS No.:149719-06-2
- H-Arg-NH2.2HCl
Catalog No.:BCC2859
CAS No.:14975-30-5
- Tereticornate A
Catalog No.:BCN1667
CAS No.:149751-81-5
- Clemastine Fumarate
Catalog No.:BCC4528
CAS No.:14976-57-9
- Delaminomycin A
Catalog No.:BCN1833
CAS No.:149779-38-4
- Homoeriodictyol 7-O-glucoside
Catalog No.:BCN7740
CAS No.:14982-11-7
- Saquinavir mesylate
Catalog No.:BCC1922
CAS No.:149845-06-7
- 2-MPMDQ
Catalog No.:BCC6741
CAS No.:149847-77-8
- AAL Toxin TB1
Catalog No.:BCN1734
CAS No.:149849-90-1
Analysis of caged xanthones from the resin of Garcinia hanburyi using ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry.[Pubmed:18940327]
Anal Chim Acta. 2008 Nov 23;629(1-2):104-18.
On-line ultra high-performance liquid chromatography (UHPLC) coupled with electrospray quadrupole time-of-flight tandem mass spectrometry (ESI-QTOF-MS/MS/MS) has been developed for the analysis of a series of caged xanthones in the resin of Garcinia hanburyi. The fragmentation of protonated molecular ions for 12 known cadged xanthones was carried out using low-energy collision-induced electrospray ionization tandem mass spectrometry. It was found that Retro-Diels-Alder rearrangement occurred in the CID processes and produced the characteristic fragment ions, which are especially valuable for the identification of this class of xanthones. The fragmentation differential between some cis-, trans-isomers was uncovered. Computation methods were utilized to rationalize the observed MS behaviors. On-line UHPLC-ESI-MS/MS/MS method has proved to be rapid and efficient in that within 6min, 15 caged scaffold xanthones, including three pairs of epimers and four pairs of isomers in gamboges, were effectively separated and identified. Among them, two known, namely isogambogenin (13) and Isomorellinol (14) and one likely new caged Garcinia xanthones from the Garcinia hanburyi were tentatively characterized based on the tandem mass spectra of known ones.
Apoptotic activity of caged xanthones from Garcinia hanburyi in cholangiocarcinoma cell lines.[Pubmed:20458760]
World J Gastroenterol. 2010 May 14;16(18):2235-43.
AIM: To investigate the growth inhibitory mechanism of four caged xanthones from Garcinia hanburyi in cholangiocarcinoma (CCA) KKU-100 and KKU-M156 cells. METHODS: Four caged xanthones, selected on the basis of their anticancer potency and chemical structure diversities (i.e. isomorellin, Isomorellinol, forbesione and gambogic acid) were used in this study. Growth inhibition of these caged xanthones was determined using the sulforhodamine B assay. Induction of apoptosis was assessed by observing cell morphology, ethidium bromide and acridine orange staining and DNA fragmentation assay. Levels of apoptotic-related gene and protein expressions were determined by a real-time reverse transcriptase polymerase chain reaction and Western blotting analysis, respectively. RESULTS: The compounds were found to inhibit growth of both cell lines in a dose-dependent manner and also showed selective cytotoxicity against the cancer cells when compared with normal peripheral blood mononuclear cells. Growth suppression by these compounds was due to apoptosis, as evidenced by the cell morphological changes, chromatin condensation, nuclear fragmentation, and DNA ladder formation. At the molecular level, these compounds induced down-regulation of Bcl-2 and survivin proteins with up-regulation of Bax and apoptosis-inducing factor proteins, leading to the activation of caspase-9 and -3 and DNA fragmentation. The functional group variations did not appear to affect the anticancer activity with regard to the two CCA cell lines; however, at a mechanistic level, Isomorellinol exhibited the highest potency in increasing the Bax/Bcl-2 protein expression ratio (120 and 41.4 for KKU-100 and KKU-M156, respectively) and in decreasing survivin protein expression (0.01 fold as compared to control cells in both cell lines). Other activities at the molecular level indicate that functional groups on the prenyl side chain may be important. CONCLUSION: Our findings for the first time demonstrate that four caged xanthones induce apoptosis in CCA cells which is mediated through a mitochondria-dependent signaling pathway.


