PoncirinCAS# 14941-08-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14941-08-3 | SDF | Download SDF |
| PubChem ID | 442456 | Appearance | Yellow powder |
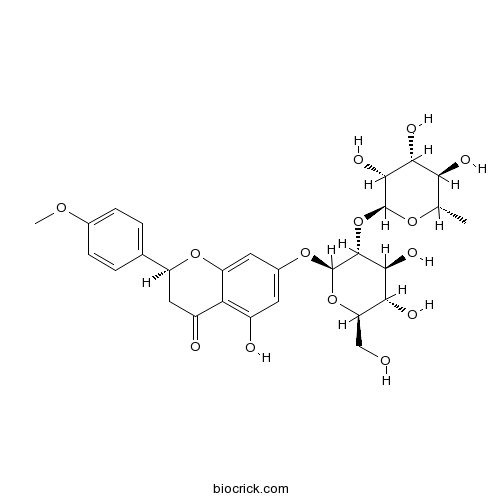
| Formula | C28H34O14 | M.Wt | 594.56 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 5,7-Dihydroxy 4'-methoxyflavanone 7-neohesperidoside; Isosakuranetin 7-neohesperidoside; 4'-O-Methylnaringin; Poncerin | ||
| Solubility | Soluble in methan | ||
| Chemical Name | (2S)-7-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-5-hydroxy-2-(4-methoxyphenyl)-2,3-dihydrochromen-4-one | ||
| SMILES | CC1C(C(C(C(O1)OC2C(C(C(OC2OC3=CC(=C4C(=O)CC(OC4=C3)C5=CC=C(C=C5)OC)O)CO)O)O)O)O)O | ||
| Standard InChIKey | NLAWPKPYBMEWIR-SKYQDXIQSA-N | ||
| Standard InChI | InChI=1S/C28H34O14/c1-11-21(32)23(34)25(36)27(38-11)42-26-24(35)22(33)19(10-29)41-28(26)39-14-7-15(30)20-16(31)9-17(40-18(20)8-14)12-3-5-13(37-2)6-4-12/h3-8,11,17,19,21-30,32-36H,9-10H2,1-2H3/t11-,17-,19+,21-,22+,23+,24-,25+,26+,27-,28+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Poncirin has anticancer, anti-bacterial and anti-inflammatory activities; it prevents adipogenesis, enhances osteoblast differentiation in mesenchymal stem cellsincreased bone mineral density, and improves trabecular microarchitecture likely reflect increases bone formation and decreases bone resorption in GIO mice. Poncirin inhibits iNOS, COX-2, TNF-alpha and IL-6 expression via the down-regulation of NF-kappaB binding activity. |
| Targets | PGE | IL Receptor | NOS | COX | TNF-α | NF-kB | p65 | PPAR |
| In vitro | Characterization, Purification of Poncirin from Edible Citrus Ougan (Citrus reticulate cv. Suavissima) and Its Growth Inhibitory Effect on Human Gastric Cancer Cells SGC-7901.[Pubmed: 23615464]Int J Mol Sci. 2013 Apr 24;14(5):8684-97.Poncirin is a bitter flavanone glycoside with various biological activities. Poncirin was isolated from four different tissues (flavedo, albedo, segment membrane, and juice sac) of Ougan fruit (Citrus reticulate cv. Suavissima). Metabolism of rutin and poncirin by human intestinal microbiota and cloning of their metabolizing α-L-rhamnosidase from Bifidobacterium dentium.[Pubmed: 25179902]J Microbiol Biotechnol. 2015 Jan 28;25(1):18-25.
Anti-Helicobacter pylori activity of the metabolites of poncirin from Poncirus trifoliata by human intestinal bacteria.[Pubmed: 10328566]Biol Pharm Bull. 1999 Apr;22(4):422-4.Poncirin was isolated from water extract of the fruits of Poncirus trifoliata and metabolized by human intestinal bacteria. |
| In vivo | Poncirin prevents bone loss in glucocorticoid-induced osteoporosis in vivo and in vitro.[Pubmed: 22407507]J Bone Miner Metab. 2012 Sep;30(5):509-16.Poncirin, a flavonoid isolated from the fruit of Poncirus trifoliata, possesses anti-bacterial and anti-inflammatory activities. However, the action of Poncirin in bone biology is unclear. In this study, the in vivo and in vitro effects of Poncirin in a glucocorticoid-induced osteoporosis (GIO) mouse model were investigated. |
| Cell Research | Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-kappaB inactivation in RAW 264.7 macrophage cells.[Pubmed: 18057724]Poncirin promotes osteoblast differentiation but inhibits adipocyte differentiation in mesenchymal stem cells.[Pubmed: 21550337]Eur J Pharmacol. 2011 Aug 16;664(1-3):54-9.Poncirin, flavanone glycoside, isolated from the fruit of Poncirus trifoliata, has anti-bacterial and anti-inflammatory activities. Biol Pharm Bull. 2007 Dec;30(12):2345-51.We previously reported that Poncirin, a flavanone glycoside isolated from the EtOAc extract of the dried immature fruits of Poncirus trifoliata, is an anti-inflammatory compound that inhibits PGE(2) and IL-6 production. |

Poncirin Dilution Calculator

Poncirin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6819 mL | 8.4096 mL | 16.8192 mL | 33.6383 mL | 42.0479 mL |
| 5 mM | 0.3364 mL | 1.6819 mL | 3.3638 mL | 6.7277 mL | 8.4096 mL |
| 10 mM | 0.1682 mL | 0.841 mL | 1.6819 mL | 3.3638 mL | 4.2048 mL |
| 50 mM | 0.0336 mL | 0.1682 mL | 0.3364 mL | 0.6728 mL | 0.841 mL |
| 100 mM | 0.0168 mL | 0.0841 mL | 0.1682 mL | 0.3364 mL | 0.4205 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- NE 100 hydrochloride
Catalog No.:BCC7573
CAS No.:149409-57-4
- Cidofovir dihydrate
Catalog No.:BCC4247
CAS No.:149394-66-1
- UNC2881
Catalog No.:BCC5362
CAS No.:1493764-08-1
- 1,2,3,4,6-O-Pentagalloylglucose
Catalog No.:BCN2338
CAS No.:14937-32-7
- UNC2250
Catalog No.:BCC4876
CAS No.:1493694-70-4
- AACOCF3
Catalog No.:BCC7075
CAS No.:149301-79-1
- Cytochalasin B
Catalog No.:BCN7084
CAS No.:14930-96-2
- pp60 c-src (521-533) (phosphorylated)
Catalog No.:BCC5851
CAS No.:149299-77-4
- 1-Dehydroxy-23-deoxojessic acid
Catalog No.:BCN1663
CAS No.:149252-87-9
- De-4'-O-methylyangambin
Catalog No.:BCN1662
CAS No.:149250-48-6
- Neotripterifordin
Catalog No.:BCN7477
CAS No.:149249-32-1
- Arcaine sulfate
Catalog No.:BCC6631
CAS No.:14923-17-2
- Traxillaside
Catalog No.:BCN6917
CAS No.:149415-62-3
- Hypocrellin C
Catalog No.:BCN3398
CAS No.:149457-83-0
- Ganoderic acid AM1
Catalog No.:BCN2441
CAS No.:149507-55-1
- Marmin
Catalog No.:BCN1665
CAS No.:14957-38-1
- Stachybotramide
Catalog No.:BCN6969
CAS No.:149598-71-0
- Impentamine dihydrobromide
Catalog No.:BCC7197
CAS No.:149629-70-9
- MOG (35-55)
Catalog No.:BCC3670
CAS No.:149635-73-4
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
- Nafadotride
Catalog No.:BCC7025
CAS No.:149649-22-9
- Chrysosplenol D
Catalog No.:BCN1666
CAS No.:14965-20-9
- Isogambogic acid
Catalog No.:BCN3078
CAS No.:149655-52-7
- Isomorellinol
Catalog No.:BCN3075
CAS No.:149655-53-8
Poncirin promotes osteoblast differentiation but inhibits adipocyte differentiation in mesenchymal stem cells.[Pubmed:21550337]
Eur J Pharmacol. 2011 Aug 16;664(1-3):54-9.
Poncirin, flavanone glycoside, isolated from the fruit of Poncirus trifoliata, has anti-bacterial and anti-inflammatory activities. In this study, the effects of Poncirin on the differentiation of mesenchymal stem cells were investigated. The C3H10T1/2 mesenchymal stem cells and primary bone marrow mesenchymal stem cells were studied. In the C3H10T1/2 cells, Poncirin prevented adipocyte differentiation, as demonstrated by inhibition of cytoplasm lipid droplet accumulation and peroxisome proliferator-activating receptor-gamma (PPAR-gamma) and CCAAT-enhancer-binding protein-beta (C/EBP-beta) mRNA expression. By contrast, Poncirin enhanced the expression of the key osteogenic transcription factors, runt-related transcription factor 2 (Runx2) and transcriptional coactivator with PDZ-binding motif (TAZ). Poncirin also enhanced expression of the osteogenic marker genes including alkaline phosphatase (ALP) and osteocalcin (OC). Poncirin increased mineral nodule formation in primary bone marrow mesenchymal stem cells. These results suggest that Poncirin prevents adipogenesis and enhances osteoblast differentiation in mesenchymal stem cells.
Poncirin prevents bone loss in glucocorticoid-induced osteoporosis in vivo and in vitro.[Pubmed:22407507]
J Bone Miner Metab. 2012 Sep;30(5):509-16.
Poncirin, a flavonoid isolated from the fruit of Poncirus trifoliata, possesses anti-bacterial and anti-inflammatory activities. However, the action of Poncirin in bone biology is unclear. In this study, the in vivo and in vitro effects of Poncirin in a glucocorticoid-induced osteoporosis (GIO) mouse model were investigated. Seven-month-old male mice were assigned to the following five groups: (1) sham-implantation (sham), (2) prednisolone 2.1 mg/kg/day (GC), (3) GC treated with 10 mg/kg/day of genistein, (4) GC treated with 3 mg/kg/day of Poncirin, (5) and GC treated with 10 mg/kg/day of strontium (GC + SrCl(2)). After 8 weeks, bone loss was measured by microcomputed tomography. Osteocalcin (OC) and C-terminal telopeptides of type I collagen (CTX) were evaluated in sera. Runx2 protein, OC and osteoprotegerin (OPG) mRNA expression, alkaline phosphatase (ALP) activity, and mineral nodule assay were performed in C3H10T1/2 or primary bone marrow stromal cells. Poncirin significantly increased the bone mineral density and improved the microarchitecture. Poncirin increased serum OC, Runx2 protein production, expression of OC and OPG mRNA, ALP activity, and mineral nodule formation; and decreased serum CTX. These effects were more prominent in the Poncirin group compared to the other positive control groups (genistein and strontium). The Poncirin-mediated restoration of biochemical bone markers, increased bone mineral density, and improved trabecular microarchitecture likely reflect increased bone formation and decreased bone resorption in GIO mice.
Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-kappaB inactivation in RAW 264.7 macrophage cells.[Pubmed:18057724]
Biol Pharm Bull. 2007 Dec;30(12):2345-51.
We previously reported that Poncirin, a flavanone glycoside isolated from the EtOAc extract of the dried immature fruits of Poncirus trifoliata, is an anti-inflammatory compound that inhibits PGE(2) and IL-6 production. The present work was undertaken to investigate the molecular actions of Poncirin in RAW 264.7 macrophage cell line. Poncirin reduced lipopolysaccharide (LPS)-induced protein levels of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) and the mRNA expressions of iNOS, COX-2, tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) in a concentration-dependent manner, as determined by Western blotting and RT-PCR, respectively. Furthermore, Poncirin inhibited the LPS-induced DNA binding activity of nuclear factor-kappaB (NF-kappaB). Moreover, this effect was accompanied by a parallel reduction in IkappaB-alpha degradation and phosphorylation that in by nuclear translocations of p50 and p65 NF-kappaB subunits. Taken together, our data indicate that anti-inflammatory properties of Poncirin might be the result from the inhibition iNOS, COX-2, TNF-alpha and IL-6 expression via the down-regulation of NF-kappaB binding activity.
Metabolism of rutin and poncirin by human intestinal microbiota and cloning of their metabolizing alpha-L-rhamnosidase from Bifidobacterium dentium.[Pubmed:25179902]
J Microbiol Biotechnol. 2015 Jan;25(1):18-25.
To understand the metabolism of flavonoid rhamnoglycosides by human intestinal microbiota, we measured the metabolic activity of rutin and Poncirin (distributed in many functional foods and herbal medicine) by 100 human stool specimens. The average alpha-Lrhamnosidase activities on the p-nitrophenyl-alpha-L-rhamnopyranoside, rutin, and Poncirin subtrates were 0.10 +/- 0.07, 0.25 +/- 0.08, and 0.15 +/- 0.09 pmol/min/mg, respectively. To investigate the enzymatic properties, alpha-L-rhamnosidase-producing bacteria were isolated from the specimens, and the alpha-L-rhamnosidase gene was cloned from a selected organism, Bifidobacterium dentium, and expressed in E. coli. The cloned alpha-L-rhamnosidase gene contained a 2,673 bp sequcence encoding 890 amino acid residues. The cloned gene was expressed using the pET 26b(+) vector in E. coli BL21, and the expressed enzyme was purified using Ni(2+)-NTA and Q-HP column chromatography. The specific activity of the purified alpha-L-rhamnosidase was 23.3 mumol/min/mg. Of the tested natural product constituents, the cloned alpha-L-rhamnosidase hydrolyzed rutin most potently, followed by Poncirin, naringin, and ginsenoside Re. However, it was unable to hydrolyze quercitrin. This is the first report describing the cloning, expression, and characterization of alpha-L-rhamnosidase, a flavonoid rhamnoglycosidemetabolizing enzyme, from bifidobacteria. Based on these findings, the alpha-L-rhamnosidase of intestinal bacteria such as B. dentium seem to be more effective in hydrolyzing (1-->6) bonds than (1-->2) bonds of rhamnoglycosides, and may play an important role in the metabolism and pharmacological effect of rhamnoglycosides.
Anti-Helicobacter pylori activity of the metabolites of poncirin from Poncirus trifoliata by human intestinal bacteria.[Pubmed:10328566]
Biol Pharm Bull. 1999 Apr;22(4):422-4.
Poncirin was isolated from water extract of the fruits of Poncirus trifoliata and metabolized by human intestinal bacteria. The inhibitory effect of Poncirin and its metabolites by these bacteria on the growth of Helicobacter pylori (HP) was investigated. Among them, ponciretin (5,7-dihydroxy-4'-methoxyflavanone), the main metabolite most potently inhibited the growth of HP, with a minimum inhibitory concentration (MIC) of 10-20 microg/ml. However, Poncirin and its metabolites except ponciretin did not inhibit the growth of HP, nor did they inhibit HP urease.
Characterization, purification of Poncirin from edible citrus Ougan (Citrus reticulate cv. Suavissima) and its growth inhibitory effect on human gastric cancer cells SGC-7901.[Pubmed:23615464]
Int J Mol Sci. 2013 Apr 24;14(5):8684-97.
Poncirin is a bitter flavanone glycoside with various biological activities. Poncirin was isolated from four different tissues (flavedo, albedo, segment membrane, and juice sac) of Ougan fruit (Citrus reticulate cv. Suavissima). The highest content of Poncirin was found in the albedo of Ougan fruit (1.37 mg/g DW). High speed counter-current chromatography (HSCCC) combined with D101 resin chromatography was utilized for the separation and purification of Poncirin from the albedo of Ougan fruit. After this two-step purification, Poncirin purity increased from 0.14% to 96.56%. The chemical structure of the purified Poncirin was identified by both HPLC-PDA and LC-MS. Poncirin showed a significant in vitro inhibitory effect on the growth of the human gastric cancer cells, SGC-7901, in a dose-dependent manner. Thus, Poncirin from Ougan fruit, may be beneficial for gastric cancer prevention. The purification method demonstrated here will be useful for further studies on the pharmacological mechanism of Poncirin activity, as well as for guiding the consumption of Ougan fruit.


