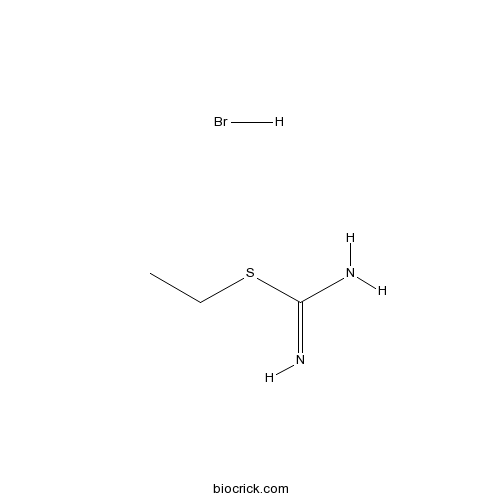
EIT hydrobromideSelective iNOS inhibitor, acts arginine binding site CAS# 1071-37-0 |
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Nelfinavir
Catalog No.:BCC4138
CAS No.:159989-64-7
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Tenofovir hydrate
Catalog No.:BCC4261
CAS No.:206184-49-8
- Dapivirine (TMC120)
Catalog No.:BCC3882
CAS No.:244767-67-7
- Zidovudine
Catalog No.:BCC5024
CAS No.:30516-87-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1071-37-0 | SDF | Download SDF |
| PubChem ID | 200213 | Appearance | Powder |
| Formula | C3H9BrN2S | M.Wt | 185.08 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | ethyl carbamimidothioate;hydrobromide | ||
| SMILES | CCSC(=N)N.Br | ||
| Standard InChIKey | SWXXKWPYNMZFTE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C3H8N2S.BrH/c1-2-6-3(4)5;/h2H2,1H3,(H3,4,5);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, selective and reversible inhibitor of isoform II NO synthase (IC50 = 13 nM; approximately 20- and 30-fold selective over isoforms I and III respectively). |

EIT hydrobromide Dilution Calculator

EIT hydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4031 mL | 27.0153 mL | 54.0307 mL | 108.0614 mL | 135.0767 mL |
| 5 mM | 1.0806 mL | 5.4031 mL | 10.8061 mL | 21.6123 mL | 27.0153 mL |
| 10 mM | 0.5403 mL | 2.7015 mL | 5.4031 mL | 10.8061 mL | 13.5077 mL |
| 50 mM | 0.1081 mL | 0.5403 mL | 1.0806 mL | 2.1612 mL | 2.7015 mL |
| 100 mM | 0.054 mL | 0.2702 mL | 0.5403 mL | 1.0806 mL | 1.3508 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- O-Phosphorylethanolamine
Catalog No.:BCN1759
CAS No.:1071-23-4
- Amyloid Beta-Peptide (12-28) (human)
Catalog No.:BCC1044
CAS No.:107015-83-8
- Granisetron HCl
Catalog No.:BCC1060
CAS No.:107007-99-8
- Sarcosine
Catalog No.:BCN2744
CAS No.:107-97-1
- H-ß-Ala-OH
Catalog No.:BCC2851
CAS No.:107-95-9
- 3-Methyl-1-butylamine
Catalog No.:BCN1810
CAS No.:107-85-7
- N-Methyltaurine
Catalog No.:BCN1751
CAS No.:107-68-6
- Betaine
Catalog No.:BCN6303
CAS No.:107-43-7
- Taurine
Catalog No.:BCN1750
CAS No.:107-35-7
- Propylamine
Catalog No.:BCN1814
CAS No.:107-10-8
- Boc-isoleucinol
Catalog No.:BCC3096
CAS No.:106946-74-1
- Adefovir
Catalog No.:BCC8808
CAS No.:106941-25-7
- Apo-12'-Lycopenal
Catalog No.:BCC8298
CAS No.:1071-52-9
- Adipic dihydrazide
Catalog No.:BCC8810
CAS No.:1071-93-8
- 8,9-Dihydroxy-10-isobutyryloxythymol
Catalog No.:BCN7974
CAS No.:107109-97-7
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Pyrocincholic acid methyl ester
Catalog No.:BCN5873
CAS No.:107160-24-7
- MAC13243
Catalog No.:BCC1727
CAS No.:1071638-38-4
- Deoxyflindissone
Catalog No.:BCN7268
CAS No.:107176-31-8
- AT-406 (SM-406)
Catalog No.:BCC1283
CAS No.:1071992-99-8
- Epigoitrin
Catalog No.:BCN6278
CAS No.:1072-93-1
- Cevimeline
Catalog No.:BCC1470
CAS No.:107233-08-9
- 2-[(1S)-2-Formyl-1,3,3-trimethylcyclohexyl]-4-hydroxy-5-propan-2-ylbenzaldehyde
Catalog No.:BCN3584
CAS No.:1072444-55-3
- NPPB
Catalog No.:BCC6711
CAS No.:107254-86-4
Effects of S-ethylisothiourea, a potent inhibitor of nitric oxide synthase, alone or in combination with a nitric oxide donor in splanchnic artery occlusion shock.[Pubmed:8872352]
Br J Pharmacol. 1996 Sep;119(1):23-8.
1. The aim of this study was to compare the effects of an intravenous infusion of a potent and non selective nitric oxide synthase inhibitor S-ethylisothiourea (Ethyl-TU) with that of a nitric oxide (NO) donor on the pathological sequelae associated with splanchnic artery occlusion (SAO) shock. In addition the effects of the combination of these two treatments were also investigated. 2. SAO shock was induced in anaesthetized rats by clamping splanchnic arteries for 45 min. Sham operated animals were used as controls. Survival time, white blood cell (WBC) count, mean arterial blood pressure, myeloperoxidase activity (MPO; studied as a quantitative means to evaluate neutrophil accumulation) and the responsiveness of aortic rings to acetylcholine (ACh, 10 nM-10 microM) and to phenylephrine (PE, 1 nM-10 microM) were studied. 3. SAO shocked rats had a decreased survival rate (0% survival 2 h after the release of occlusion) and survival time (76 +/- 10 min), increased MPO activity in the ileum (3.39 +/- 0.8 u x 10(-3) g-1 tissue), a marked leukopenia and a profound hypotension. In addition aortic rings from shocked rats showed a marked hyporeactivity to PE and reduced responsiveness to ACh. Endothelium denuded aortic rings had also a marked hyporeactivity to PE. 4. In vivo administration of Ethyl-TU (0.1 mg kg-1 h-1, beginning 1 min after the onset of reperfusion) significantly increased survival time and rate, improved mean arterial blood pressure, restored the responsiveness to PE, but did not change MPO activity, leukopenia or the impairment in the responsiveness of aortic rings to ACh. Addition of Ethyl-TU (2 microM) to endothelium denuded aortic rings in vitro, restored the marked hyporeactivity to PE. Administration of the NO donor C87-3754 (0.75 mg kg-1 h-1, beginning 1 min after the onset of reperfusion) slightly increased survival time and reduced MPO activity and leukopenia, but did not change survival rate and mean arterial blood pressure. In addition C87-3754 restored the responsiveness of aortic rings to ACh to control values, but did not modify the hyporeactivity to PE. The combination of these two interventions produced a higher degree of protection than either Ethyl-TU or C87-3754 alone. In fact, co-administration of Ethyl-TU plus C87-3754 completely prevented mortality, reduced MPO activity, attenuated leukopenia and the profound hypotension and restored the impaired responsiveness of aortic rings to PE and ACh. 5. Our study suggests that treatment with a nitric oxide synthase inhibitor combined with an NO donor may be a new therapeutic approach to the treatment of splanchnic artery occlusion shock.
Novel potent and selective inhibitors of inducible nitric oxide synthase.[Pubmed:7536889]
Mol Pharmacol. 1995 Apr;47(4):831-4.
We have identified two novel potent and selective inhibitors of inducible nitric oxide synthase, S-ethylisothiourea and 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine. Ki values of 14.7 nM for S-ethylisothiourea and 4.2 nM for 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine were obtained with partially purified preparations of inducible nitric oxide synthase. These compounds demonstrate about 1000-fold greater potency than prototypical inhibitors, and the inhibitions are 10-40-fold more selective for murine inducible nitric oxide synthase, compared with the rat neuronal and bovine endothelial isoforms of nitric oxide synthase. These compounds also potently inhibit the nitric oxide synthase activity in intact J774 mouse macrophages. The inhibition is competitive with the substrate L-arginine and reversible in both enzymatic and intact cell assays. These potent and selective inhibitors of inducible nitric oxide synthase may have potential therapeutic applications in the treatment of inflammatory and autoimmune diseases.
Potent and selective inhibition of human nitric oxide synthases. Inhibition by non-amino acid isothioureas.[Pubmed:7523409]
J Biol Chem. 1994 Oct 28;269(43):26669-76.
S-Ethylisothiourea was a potent competitive inhibitor of human nitric oxide synthase (NOS), with Ki values of 17, 36, and 29 nM for the inducible (i), endothelial (e), and neuronal (n) isozymes, respectively. Unlike some potent inhibitors of NOS, no time dependence was observed. S-Ethylisothiourea was not a detectable substrate for eNOS. S-Ethylisothiourea was also a potent inhibitor of mouse iNOS (Ki value of 5.2 nM), and its binding perturbed the spectrum of iNOS consistent with its altering the environment of the bound heme. The optimum binding of S-ethyl- and S-isopropylisothiourea relative to 70 other analogs suggested that these alkyl substitutions fit into a small hydrophobic pocket. Most isothioureas were 2-6-fold selective for the human iNOS (Ki for iNOS versus Ki for eNOS), with one being 19-fold selective. The cyclized mimics of S-ethylisothiourea, 2-NH2-thiazoline, and 2-NH2-thiazole, were also competitive inhibitors of human NOS. A third structural class of inhibitors, bisisothioureas, were, in general, the most selective in their inhibition of human iNOS. S,S'-(1,3-Phenylenebis(1,2-ethanediyl))bisisothiourea was 190-fold selective (Ki value of 0.047 microM against iNOS versus 9.0 microM against eNOS). These results demonstrate that potent and selective inhibition of human NOS isozymes is achievable.


