Perindopril ErbumineACE inhibitor CAS# 107133-36-8 |
- Losartan Potassium (DuP 753)
Catalog No.:BCC1080
CAS No.:124750-99-8
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Telmisattan
Catalog No.:BCC3863
CAS No.:144701-48-4
- Rosuvastatin Calcium
Catalog No.:BCC3853
CAS No.:147098-20-2
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 107133-36-8 | SDF | Download SDF |
| PubChem ID | 441313 | Appearance | Powder |
| Formula | C23H43N3O5 | M.Wt | 441.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Perindopril tert-butylamine salt | ||
| Solubility | H2O : ≥ 50 mg/mL (113.22 mM) DMSO : 10 mg/mL (22.64 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
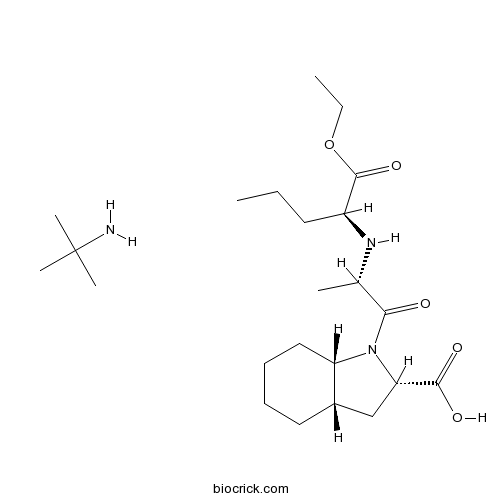
| Chemical Name | (2S,3aS,7aS)-1-[(2S)-2-[[(2S)-1-ethoxy-1-oxopentan-2-yl]amino]propanoyl]-2,3,3a,4,5,6,7,7a-octahydroindole-2-carboxylic acid;2-methylpropan-2-amine | ||
| SMILES | CCCC(C(=O)OCC)NC(C)C(=O)N1C2CCCCC2CC1C(=O)O.CC(C)(C)N | ||
| Standard InChIKey | IYNMDWMQHSMDDE-MHXJNQAMSA-N | ||
| Standard InChI | InChI=1S/C19H32N2O5.C4H11N/c1-4-8-14(19(25)26-5-2)20-12(3)17(22)21-15-10-7-6-9-13(15)11-16(21)18(23)24;1-4(2,3)5/h12-16,20H,4-11H2,1-3H3,(H,23,24);5H2,1-3H3/t12-,13-,14-,15-,16-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Angiotensin-converting enzyme (ACE) inhibitor (IC50 = 1.05 nM). Brain penetrant. Suppresses the increase in hippocampal ACE activity and improves cognition in PS2APP-transgenic mice. |

Perindopril Erbumine Dilution Calculator

Perindopril Erbumine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2645 mL | 11.3225 mL | 22.6449 mL | 45.2899 mL | 56.6123 mL |
| 5 mM | 0.4529 mL | 2.2645 mL | 4.529 mL | 9.058 mL | 11.3225 mL |
| 10 mM | 0.2264 mL | 1.1322 mL | 2.2645 mL | 4.529 mL | 5.6612 mL |
| 50 mM | 0.0453 mL | 0.2264 mL | 0.4529 mL | 0.9058 mL | 1.1322 mL |
| 100 mM | 0.0226 mL | 0.1132 mL | 0.2264 mL | 0.4529 mL | 0.5661 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Angiotensin-converting enzyme (ACE) inhibitor (IC50 = 1.05 nM). Brain penetrant. Suppresses the increase in hippocampal ACE activity and improves cognition in PS2APP-transgenic mice.
- 8,9-Dihydroxy-10-isobutyryloxythymol
Catalog No.:BCN7974
CAS No.:107109-97-7
- Adipic dihydrazide
Catalog No.:BCC8810
CAS No.:1071-93-8
- Apo-12'-Lycopenal
Catalog No.:BCC8298
CAS No.:1071-52-9
- EIT hydrobromide
Catalog No.:BCC6824
CAS No.:1071-37-0
- O-Phosphorylethanolamine
Catalog No.:BCN1759
CAS No.:1071-23-4
- Amyloid Beta-Peptide (12-28) (human)
Catalog No.:BCC1044
CAS No.:107015-83-8
- Granisetron HCl
Catalog No.:BCC1060
CAS No.:107007-99-8
- Sarcosine
Catalog No.:BCN2744
CAS No.:107-97-1
- H-ß-Ala-OH
Catalog No.:BCC2851
CAS No.:107-95-9
- 3-Methyl-1-butylamine
Catalog No.:BCN1810
CAS No.:107-85-7
- N-Methyltaurine
Catalog No.:BCN1751
CAS No.:107-68-6
- Betaine
Catalog No.:BCN6303
CAS No.:107-43-7
- Pyrocincholic acid methyl ester
Catalog No.:BCN5873
CAS No.:107160-24-7
- MAC13243
Catalog No.:BCC1727
CAS No.:1071638-38-4
- Deoxyflindissone
Catalog No.:BCN7268
CAS No.:107176-31-8
- AT-406 (SM-406)
Catalog No.:BCC1283
CAS No.:1071992-99-8
- Epigoitrin
Catalog No.:BCN6278
CAS No.:1072-93-1
- Cevimeline
Catalog No.:BCC1470
CAS No.:107233-08-9
- 2-[(1S)-2-Formyl-1,3,3-trimethylcyclohexyl]-4-hydroxy-5-propan-2-ylbenzaldehyde
Catalog No.:BCN3584
CAS No.:1072444-55-3
- NPPB
Catalog No.:BCC6711
CAS No.:107254-86-4
- Baogongteng C
Catalog No.:BCN1873
CAS No.:107259-50-7
- Carasinol D
Catalog No.:BCN8228
CAS No.:1072797-66-0
- MLN2238
Catalog No.:BCC2092
CAS No.:1072833-77-2
- SR-3677
Catalog No.:BCC4302
CAS No.:1072959-67-1
Novel Spectrofluorimetric Method for the Determination of Perindopril Erbumine Based on Charge Transfer Reaction with 7-Hydroxycoumarin.[Pubmed:26149499]
J Fluoresc. 2015 Jul;25(4):811-8.
A novel, simple, selective and sensitive spectrofluorimetric method was developed and validated for the determination of Perindopril Erbumine using 7-hydroxycoumarin. Perindopril Erbumine was found to react with 7-hydroxycoumarin in acetonitrile resulting in a new fluorescent product with about 58 nm blue shifted emission. The fluorescence of the complex was measured at 440 nm after excitation at 350 nm in acetonitrile. Under the optimum conditions, the fluorescence intensity was linear over a concentration range of 2.0-16.0 mug/mL (R(2) = 1) with a detection limit of 0.054 mug/mL. The proposed method was fully validated and successfully applied to the analysis of Perindopril Erbumine in pure form and tablets. Statistical comparison of the results obtained by the proposed and reference method revealed no significant differences in the performance of the two methods regarding the accuracy and precision respectively. The method was shown to be highly specific in the presence of indapamide, a diuretic that is commonly combined with Perindopril Erbumine. A proposal for the reaction pathway with 7-hydroxycoumarin was postulated.
Novel Spectrofluorimetric Method for the Determination of Perindopril Erbumine Based on Fluorescence Quenching of Rhodamine B.[Pubmed:26438658]
J Fluoresc. 2015 Nov;25(6):1577-84.
A novel, simple and specific spectrofluorimetric method was developed and validated for the determination of Perindopril Erbumine (PDE). The method is based on the fluorescence quenching of Rhodamine B upon adding Perindopril Erbumine. The quenched fluorescence was monitored at 578 nm after excitation at 500 nm. The optimization of the reaction conditions such as the solvent, reagent concentration, and reaction time were investigated. Under the optimum conditions, the fluorescence quenching was linear over a concentration range of 1.0-6.0 mug/mL. The proposed method was fully validated and successfully applied to the analysis of Perindopril Erbumine in pure form and tablets. Statistical comparison of the results obtained by the developed and reference methods revealed no significant differences between the methods compared in terms of accuracy and precision. The method was shown to be highly specific in the presence of indapamide, a diuretic that is commonly combined with Perindopril Erbumine. The mechanism of rhodamine B quenching was also discussed.
Influence of different types of commercially available microcrystalline cellulose on degradation of perindopril erbumine and enalapril maleate in binary mixtures.[Pubmed:23333887]
Acta Pharm. 2012 Dec;62(4):515-28.
Influence of some commercially available types of microcrystalline cellulose (MCC) on the stability of certain active pharmaceutical ingredients (APIs), when in contact, has been investigated. Two structurally similar APIs, Perindopril Erbumine (PER) and enalapril maleate (EM), both well-known angiotensin-converting enzyme inhibitors were used. The main properties of an MCC that could determine the stability for each API were measured and correlated to the stability of these two APIs in binary mixtures. The stability of these APIs differed when in contact with different types of MCC. The dominant properties of MCC from one manufacturer were surface features that influenced the stability of PER and acidity that influenced the stability of EM. In the case of MCC from other manufacturers, unbound water was stability determining for both substances.
Stability-indicating RP-HPLC method for the quantitative analysis of perindopril erbumine in tablet dosage form.[Pubmed:23690066]
J Chromatogr Sci. 2014 Apr;52(4):315-20.
A specific, stability-indicating reversed-phase high-performance liquid chromatography (RP-HPLC) method was developed and validated for the estimation of Perindopril Erbumine (PDE) in tablet dosage form. The HPLC method showed adequate separation of PDE from its degradation products. The separation was achieved on a Phenomenex Luna C18 column (250 x 4.6 mm x 5 microm) using a mobile phase composition of 0.2% trifluoroacetic acid buffer and acetonitrile in the ratio of 60:40 (pH adjusted to 3 with ammonia) at a flow rate of 1 mL/min. The injection volume was 20 microL and the wavelength of detection was kept at 215 nm. Stress studies were performed with 1 mg/mL of each drug, starting with mild conditions and followed by stronger conditions to achieve sufficient degradation at approximately 5-20%. The linearity of the proposed method was investigated in the range of 2.5 to 50 microg/mL for PDE. The limits of detection and quantification were found to be 0.75 and 2.3 microg/mL, respectively.
Perindopril, a centrally active angiotensin-converting enzyme inhibitor, prevents cognitive impairment in mouse models of Alzheimer's disease.[Pubmed:21593435]
FASEB J. 2011 Sep;25(9):2911-20.
The purpose of this work was to test whether brain-penetrating angiotensin-converting enzyme (ACE) inhibitors (e.g., perindopril), as opposed to non-brain-penetrating ACE inhibitors (e.g., enalapril and imidapril), may reduce the cognitive decline and brain injury in Alzheimer's disease (AD). We first compared the effect of perindopril, enalapril, and imidapril on cognitive impairment and brain injury in a mouse model of AD induced by intracerebroventricular (i.c.v.) injection of amyloid-beta (Abeta)(1)(-)(4)(0). Perindopril, with significant inhibition of hippocampal ACE, significantly prevented cognitive impairment in this AD mouse model. This beneficial effect was attributed to the suppression of microglia/astrocyte activation and the attenuation of oxidative stress caused by iNOS induction and extracellular superoxide dismutase down-regulation. In contrast, neither enalapril nor imidapril prevented cognitive impairment and brain injury in this AD mouse. We next examined the protective effects of perindopril on cognitive impairment in PS2APP-transgenic mice overexpressing Abeta in the brain. Perindopril, without affecting brain Abeta deposition, significantly suppressed the increase in hippocampal ACE activity and improved cognition in PS2APP-transgenic mice, being associated with the suppression of hippocampal astrocyte activation and attenuation of superoxide. Our data demonstrated that the brain-penetrating ACE inhibitor perindopril, as compared to non-brain-penetrating ACE inhibitors, protected against cognitive impairment and brain injury in experimental AD models.
Angiotensin-converting enzyme (ACE) inhibitors have different selectivity for bradykinin binding sites of human somatic ACE.[Pubmed:17716647]
Eur J Pharmacol. 2007 Dec 22;577(1-3):1-6.
The angiotensin-converting enzyme (ACE) has two natural substrates and two catalytic domains: one cleaving angiotensin I and one inactivating bradykinin. The aim of this study was to investigate the comparative binding affinity of ACE inhibitors for the two binding sites of human endothelial ACE. In vitro binding assays were performed to test the ability of bradykinin, angiotensin I, or various ACE inhibitors (enalaprilat, perindoprilat, quinaprilat, ramiprilat, and trandolaprilat) to displace a saturating concentration of [(125)I]351A, a radiolabeled lisinopril analogue, from ACE binding sites. The calculated IC(50) values for the ACE inhibitors were in the nanomolar range, while those for the natural substrates were in the micromolar range. The bradykinin/angiotensin I selectivity ratios calculated from double displacement experiments were: perindoprilat, 1.44; ramiprilat, 1.16; quinaprilat, 1.09; trandolaprilat, 1.08; enalaprilat, 1.00. The ACE inhibitors generally had higher affinity for the bradykinin than the angiotensin I binding sites, supporting the idea that these agents are primarily inhibitors of bradykinin degradation, and secondarily inhibitors of angiotensin II production. Perindoprilat had the highest selectivity for bradykinin versus angiotensin I binding sites, and enalaprilat has the lowest. These results indicate that there are differences in the affinity of ACE inhibitors toward sites for bradykinin degradation, which could lead to differences in efficacy in cardiovascular disease.
Significant target organs for hypertension and cardiac hypertrophy by angiotensin-converting enzyme inhibitors.[Pubmed:15080380]
Hypertens Res. 2004 Mar;27(3):213-9.
To clarify the mechanisms by which angiotensin-converting enzyme (ACE) inhibitors lower blood pressure or inhibit cardiac hypertrophy, we analyzed the correlations among tissue ACE activities, blood pressure and cardiac hypertrophy. In spontaneously hypertensive rats (SHR), blood pressure, heart weight and ACE activities in plasma and various tissues were measured 3, 24 and 48 h after repeated daily treatment for 2 weeks with the ACE inhibitors trandolapril, perindopril, temocapril and enalapril. For all four ACE inhibitors, blood pressure and ACE activities in the plasma, aorta and kidney were significantly reduced 3 h after the last treatment. Although hypotensive effects were maintained at 24 h, ACE activities in plasma were not suppressed by temocapril and enalapril. Even at 3 h, enalapril could not suppress ACE activity in the brain, and temocapril and enalapril could not inhibit ACE activities in the heart. Significant correlations between ACE activity in the aorta and blood pressure were observed for all four ACE inhibitors, while the ACE activities in the heart and brain were not correlated with changes in blood pressure. Significant decreases in the ratio of heart weight to body weight were observed in SHR treated with trandolapril and perindopril, whereas they were not observed with temocapril and enalapril. The ratio of heart weight to body weight was significantly correlated with ACE activity in the heart. ACE activities in vascular tissues and the heart may be important targets in terms of the ability of ACE inhibitors to lower blood pressure or inhibit cardiac hypertrophy, respectively.


