O-PhosphorylethanolamineCAS# 1071-23-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1071-23-4 | SDF | Download SDF |
| PubChem ID | 1015 | Appearance | Powder |
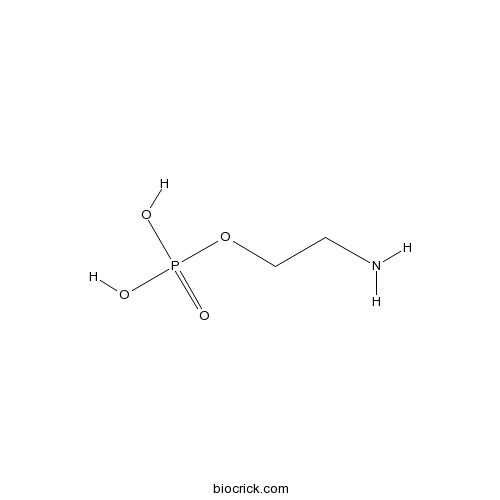
| Formula | C2H8NO4P | M.Wt | 141.1 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | 2-Aminoethyl dihydrogen phosphate | ||
| Solubility | H2O : 250 mg/mL (1772.30 mM; Need ultrasonic) | ||
| Chemical Name | 2-AMINOETHYL DIHYDROGEN PHOSPHATE | ||
| SMILES | NCCO[P](O)(O)=O | ||
| Standard InChIKey | SUHOOTKUPISOBE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C2H8NO4P/c3-1-2-7-8(4,5)6/h1-3H2,(H2,4,5,6) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. O-Phosphorylethanolamine coupled with aminosilanized nanodiamonds show a homogeneous interaction with the titanium substrate. |

O-Phosphorylethanolamine Dilution Calculator

O-Phosphorylethanolamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.0872 mL | 35.4359 mL | 70.8717 mL | 141.7434 mL | 177.1793 mL |
| 5 mM | 1.4174 mL | 7.0872 mL | 14.1743 mL | 28.3487 mL | 35.4359 mL |
| 10 mM | 0.7087 mL | 3.5436 mL | 7.0872 mL | 14.1743 mL | 17.7179 mL |
| 50 mM | 0.1417 mL | 0.7087 mL | 1.4174 mL | 2.8349 mL | 3.5436 mL |
| 100 mM | 0.0709 mL | 0.3544 mL | 0.7087 mL | 1.4174 mL | 1.7718 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Amyloid Beta-Peptide (12-28) (human)
Catalog No.:BCC1044
CAS No.:107015-83-8
- Granisetron HCl
Catalog No.:BCC1060
CAS No.:107007-99-8
- Sarcosine
Catalog No.:BCN2744
CAS No.:107-97-1
- H-ß-Ala-OH
Catalog No.:BCC2851
CAS No.:107-95-9
- 3-Methyl-1-butylamine
Catalog No.:BCN1810
CAS No.:107-85-7
- N-Methyltaurine
Catalog No.:BCN1751
CAS No.:107-68-6
- Betaine
Catalog No.:BCN6303
CAS No.:107-43-7
- Taurine
Catalog No.:BCN1750
CAS No.:107-35-7
- Propylamine
Catalog No.:BCN1814
CAS No.:107-10-8
- Boc-isoleucinol
Catalog No.:BCC3096
CAS No.:106946-74-1
- Adefovir
Catalog No.:BCC8808
CAS No.:106941-25-7
- Boc-D-Leucinol
Catalog No.:BCC2723
CAS No.:106930-51-2
- EIT hydrobromide
Catalog No.:BCC6824
CAS No.:1071-37-0
- Apo-12'-Lycopenal
Catalog No.:BCC8298
CAS No.:1071-52-9
- Adipic dihydrazide
Catalog No.:BCC8810
CAS No.:1071-93-8
- 8,9-Dihydroxy-10-isobutyryloxythymol
Catalog No.:BCN7974
CAS No.:107109-97-7
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Pyrocincholic acid methyl ester
Catalog No.:BCN5873
CAS No.:107160-24-7
- MAC13243
Catalog No.:BCC1727
CAS No.:1071638-38-4
- Deoxyflindissone
Catalog No.:BCN7268
CAS No.:107176-31-8
- AT-406 (SM-406)
Catalog No.:BCC1283
CAS No.:1071992-99-8
- Epigoitrin
Catalog No.:BCN6278
CAS No.:1072-93-1
- Cevimeline
Catalog No.:BCC1470
CAS No.:107233-08-9
- 2-[(1S)-2-Formyl-1,3,3-trimethylcyclohexyl]-4-hydroxy-5-propan-2-ylbenzaldehyde
Catalog No.:BCN3584
CAS No.:1072444-55-3
Detonation nanodiamonds biofunctionalization and immobilization to titanium alloy surfaces as first steps towards medical application.[Pubmed:25550742]
Beilstein J Org Chem. 2014 Nov 26;10:2765-2773.
Due to their outstanding properties nanodiamonds are a promising nanoscale material in various applications such as microelectronics, polishing, optical monitoring, medicine and biotechnology. Beyond the typical diamond characteristics like extreme hardness or high thermal conductivity, they have additional benefits as intrinsic fluorescence due to lattice defects without photobleaching, obtained during the high pressure high temperature process. Further the carbon surface and its various functional groups in consequence of the synthesis, facilitate additional chemical and biological modification. In this work we present our recent results on chemical modification of the nanodiamond surface with phosphate groups and their electrochemically assisted immobilization on titanium-based materials to increase adhesion at biomaterial surfaces. The starting material is detonation nanodiamond, which exhibits a heterogeneous surface due to the functional groups resulting from the nitrogen-rich explosives and the subsequent purification steps after detonation synthesis. Nanodiamond surfaces are chemically homogenized before proceeding with further functionalization. Suspensions of resulting surface-modified nanodiamonds are applied to the titanium alloy surfaces and the nanodiamonds subsequently fixed by electrochemical immobilization. Titanium and its alloys have been widely used in bone and dental implants for being a metal that is biocompatible with body tissues and able to bind with adjacent bone during healing. In order to improve titanium material properties towards biomedical applications the authors aim to increase adhesion to bone material by incorporating nanodiamonds into the implant surface, namely the anodically grown titanium dioxide layer. Differently functionalized nanodiamonds are characterized by infrared spectroscopy and the modified titanium alloys surfaces by scanning and transmission electron microscopy. The process described shows an adsorption and immobilization of modified nanodiamonds on titanium; where aminosilanized nanodiamonds coupled with O-Phosphorylethanolamine show a homogeneous interaction with the titanium substrate.


