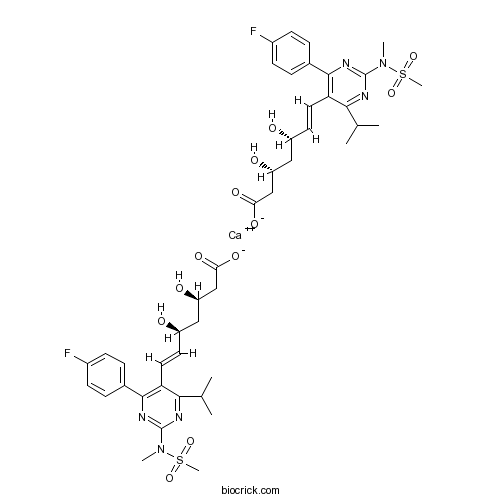
Rosuvastatin CalciumCAS# 147098-20-2 |
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Losartan Potassium (DuP 753)
Catalog No.:BCC1080
CAS No.:124750-99-8
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Telmisattan
Catalog No.:BCC3863
CAS No.:144701-48-4
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 147098-20-2 | SDF | Download SDF |
| PubChem ID | 5282455 | Appearance | Powder |
| Formula | C44H54F2N6O12S2 Ca | M.Wt | 1001.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Rosuvastatin hemicalcium; ZD 4522 Calcium | ||
| Solubility | DMSO : 25 mg/mL (49.94 mM; Need ultrasonic) H2O : 1 mg/mL (2.00 mM; Need ultrasonic) | ||
| Chemical Name | calcium;(E,3R,5S)-7-[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan-2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate | ||
| SMILES | [Ca++].CC(C)c1nc(nc(c2ccc(F)cc2)c1C=C[C@@H](O)C[C@@H](O)CC([O-])=O)N(C)[S](C)(=O)=O.CC(C)c3nc(nc(c4ccc(F)cc4)c3/C=C/[C@@H](O)C[C@@H](O)CC([O-])=O)N(C)[S](C)(=O)=O | ||
| Standard InChIKey | LALFOYNTGMUKGG-BGRFNVSISA-L | ||
| Standard InChI | InChI=1S/2C22H28FN3O6S.Ca/c2*1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32;/h2*5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30);/q;;+2/p-2/b2*10-9+;/t2*16-,17-;/m11./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent HMG-CoA reductase inhibitor (IC50 = 5.4 nM). Inhibits cholesterol synthesis in rat hepatocytes in vitro and in vivo. Reduces plasma LDL cholesterol levels. Orally bioavailable. |

Rosuvastatin Calcium Dilution Calculator

Rosuvastatin Calcium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.9989 mL | 4.9943 mL | 9.9886 mL | 19.9772 mL | 24.9715 mL |
| 5 mM | 0.1998 mL | 0.9989 mL | 1.9977 mL | 3.9954 mL | 4.9943 mL |
| 10 mM | 0.0999 mL | 0.4994 mL | 0.9989 mL | 1.9977 mL | 2.4972 mL |
| 50 mM | 0.02 mL | 0.0999 mL | 0.1998 mL | 0.3995 mL | 0.4994 mL |
| 100 mM | 0.01 mL | 0.0499 mL | 0.0999 mL | 0.1998 mL | 0.2497 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A selective, competitive inhibitor of HMG-CoA reductase, that is also antilipemic.
- 5,6-Dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-4-one
Catalog No.:BCC8722
CAS No.:147086-79-1
- Alcaftadine
Catalog No.:BCC5260
CAS No.:147084-10-4
- Trovafloxacin mesylate
Catalog No.:BCC3931
CAS No.:147059-75-4
- Rocaglaol
Catalog No.:BCN1653
CAS No.:147059-46-9
- Cyclo(Phe-Pro)
Catalog No.:BCN2416
CAS No.:14705-60-3
- MK591
Catalog No.:BCC1766
CAS No.:147030-01-1
- Menthyl-5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-[1,3]oxathiolane-2-carboxylic acid
Catalog No.:BCC9019
CAS No.:147027-10-9
- 3'-O-Demethylarctigenin
Catalog No.:BCN3544
CAS No.:147022-95-5
- Cytarabine
Catalog No.:BCC3759
CAS No.:147-94-4
- Proline
Catalog No.:BCN1656
CAS No.:147-85-3
- DL-Arabinose
Catalog No.:BCN8541
CAS No.:147-81-9
- Diphenhydramine hydrochloride
Catalog No.:BCC8947
CAS No.:147-24-0
- Maropitant
Catalog No.:BCC1728
CAS No.:147116-67-4
- Methyl (3R)-3-(tert-butyldimethylsilyloxy)-5-oxo-6-triphenylphosphoranylidenehexanoate
Catalog No.:BCC9031
CAS No.:147118-35-2
- 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidinyl-5-yl-formyl
Catalog No.:BCC8651
CAS No.:147118-37-4
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- 4-Chloro-L-phenylalanine Hydrochloride
Catalog No.:BCC2638
CAS No.:123053-23-6
- TT 232
Catalog No.:BCC6248
CAS No.:147159-51-1
- N-6-Methyl-7,7-dioxo-2-sulfamoyl-5,6-dihydro-4H-thieno[2,3-b]thiopyran-4-yl]acetamide
Catalog No.:BCC9077
CAS No.:147200-03-1
- Arecaidine propargyl ester tosylate
Catalog No.:BCC6628
CAS No.:147202-94-6
- Delavirdine mesylate
Catalog No.:BCC4069
CAS No.:147221-93-0
- glatiramer acetate
Catalog No.:BCC5642
CAS No.:147245-92-9
- 7ACC2
Catalog No.:BCC5554
CAS No.:1472624-85-3
- KRCA 0008
Catalog No.:BCC8007
CAS No.:1472795-20-2
Solidified self nano-emulsifying drug delivery system of rosuvastatin calcium to treat diet-induced hyperlipidemia in rat: in vitro and in vivo evaluations.[Pubmed:28145826]
Ther Deliv. 2017 Jan;8(3):125-136.
The present work focuses on preparing a solidified self nano-emulsifying drug-delivery system (S-SNEDDS) to improve the in vitro dissolution of rosuvastatin and to evaluate its antihyperlipidemic activity. Powder flow characterization demonstrated good flow properties. The drug-excipient compatibility study indicates no possible interaction. Transmission electron microscopy and scanning electron microscopy revealed nonaggregated, spherical nanosized globules. The globule-size analysis revealed droplet size in nanorange ( approximately 100 nm). S-SNEDDS exhibited improved drug release ( approximately 95%) as compared with rosuvastatin powder (51.89%) at 60 min. Upon antihyperlipidemic study, S-SNEDDS after 14th day of treatment revealed significant reduction in cholesterol (33.47%), triglycerides (40.77%) and atherogenic index (81.28%), while high-density lipoprotein (118.43%) was increased. The study indicates the great potential of S-SNEDDS for improving oral absorption of such poorly soluble drugs and their pharmacodynamic efficacy.
Improved anti-hyperlipidemic activity of Rosuvastatin Calcium via lipid nanoparticles: Pharmacokinetic and pharmacodynamic evaluation.[Pubmed:27810472]
Eur J Pharm Biopharm. 2017 Jan;110:47-57.
The intent of this investigation was to improve pharmacokinetic (PK) and pharmacodynamic (PD) effects of Rosuvastatin Calcium (RC) by solid lipid nanoparticles (SLNs). RC is anti-hyperlipidemic drug with low oral bioavailability (20%) due to first-pass metabolism. Hot homogenization followed by ultrasonication method was used to prepare RC-SLNs with stearic acid, glyceryl behenate and glyceryl trilaurate as lipid matrices, egg lecithin and poloxamer 188 as surfactants. The prepared SLNs were tested for particle size, PDI, zeta potential (ZP), entrapment efficiency (EE), drug content and in vitro release. Further, PK and PD studies were conducted on selected SLNs. No changes in physical stability of the optimized SLN were observed at refrigerated and room temperature for 90days. SLNs prepared with glyceryl trilaurate having average size of 67.21+/-1.71nm, PDI of 0.25+/-0.01, ZP of -28.93+/-0.84mV with 93.51+/-0.34% EE was considered as optimized. DSC and XRD studies revealed that no interaction occurred between the drug and lipid. SEM and TEM studies revealed that SLNs were nearly spherical in shape. PK studies showed improvement in the oral bioavailability (extent of absorption) of SLNs by 4.6-fold when compared to that of suspension. PD study of SLNs in hyperlipidemic rats exhibited a decrease in lipid profile for 36h, while a suspension exhibited for 24h.
Pharmacokinetic and bioequivalence study comparing a candesartan cilexetil/rosuvastatin calcium fixed-dose combination with the concomitant administration of candesartan cilexetil and rosuvastatin calcium in healthy Korean subjects.[Pubmed:28079517]
Int J Clin Pharmacol Ther. 2017 Mar;55(3):286-294.
CONTEXT: A fixed-dose combination (FDC) of candesartan and rosuvastatin was recently developed for the treatment of cardiovascular disease and expected to enhance patient compliance. OBJECTIVE: This study was performed to compare the single-dose pharmacokinetic properties and tolerability of DP-R208 (candesartan and rosuvastatin FDC) to those of each component administered alone in healthy Korean male volunteers. MATERIALS AND METHODS: A total of 40 healthy Korean volunteers were enrolled in this randomized, open-label, single-dose, two-treatment, two-way crossover study. During each treatment period, subjects received the test formulation (FDC tablet containing candesartan and rosuvastatin) or reference formulation (co-administration of candesartan and rosuvastatin). Plasma samples were collected pre-dose and at 0.5, 1, 2, 3, 4, 5, 6, 8, 12, 24, and 48 hours post-dose. Safety and tolerability were assessed by the evaluation of adverse events (AEs), physical examinations, laboratory assessments, 12-lead electrocardiograms (ECGs), and vital sign measurements. RESULTS: The 90% confidence intervals (CIs) of the geometric least-square mean ratios of Cmax, AUClast, and AUCinf were 0.86 - 1.00, 0.92 - 1.04, and 0.92 - 1.03 for candesartan, and 0.88 - 1.06, 0.91 - 1.08, and 0.91 - 1.03 for rosuvastatin, respectively. All of the AEs were mild, and there was no significant difference in the incidence of AEs between the formulations. Furthermore, the pharmacokinetic properties of the test and reference formulations met the regulatory criteria for bioequivalence. Discussion and conclusion: Both formulations were safe and well tolerated, and no significant difference was observed in the safety assessments of the treatments..
Novel surface-engineered solid lipid nanoparticles of rosuvastatin calcium for low-density lipoprotein-receptor targeting: a Quality by Design-driven perspective.[Pubmed:28093941]
Nanomedicine (Lond). 2017 Feb;12(4):333-356.
AIM: The present studies describe Quality by Design-oriented development and characterization of surface-engineered solid lipid nanoparticles (SLNs) of Rosuvastatin Calcium for low density lipoprotein-receptor targeting. MATERIALS & METHODS: SLNs were systematically prepared employing Compritol 888 and Tween-80. Surface modification of SLNs was accomplished with Phospholipon 90G and DSPE-mPEG-2000 as the ligands for specific targeting to the low density lipoprotein-receptors. SLNs were evaluated for size, potential, entrapment, drug release performance and gastric stability. Also, the formulations were evaluated for cellular cytotoxicity, uptake and permeability, pharmacokinetic, pharmacodynamic and biochemical studies. RESULTS & CONCLUSION: Overall, the studies ratified enhanced biopharmaceutical performance of the surface-engineered SLNs of rosuvastatin as a novel approach for the management of hyperlipidemia-like conditions.


