AlcaftadineCAS# 147084-10-4 |
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- PA-824
Catalog No.:BCC1106
CAS No.:187235-37-6
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 147084-10-4 | SDF | Download SDF |
| PubChem ID | 19371515 | Appearance | Powder |
| Formula | C19H21N3O | M.Wt | 307.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | R89674 | ||
| Solubility | DMSO : ≥ 40 mg/mL (130.13 mM) *"≥" means soluble, but saturation unknown. | ||
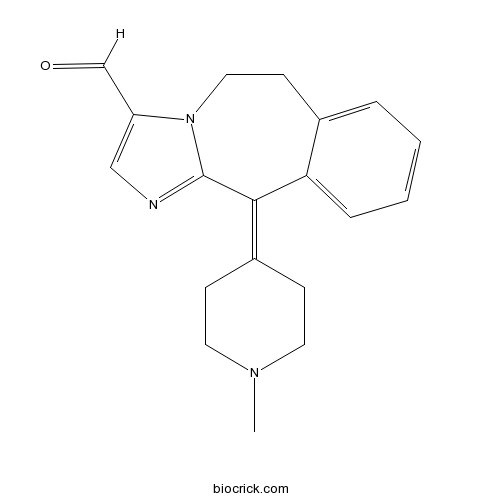
| Chemical Name | 11-(1-methylpiperidin-4-ylidene)-5,6-dihydroimidazo[2,1-b][3]benzazepine-3-carbaldehyde | ||
| SMILES | CN1CCC(=C2C3=CC=CC=C3CCN4C2=NC=C4C=O)CC1 | ||
| Standard InChIKey | MWTBKTRZPHJQLH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H21N3O/c1-21-9-6-15(7-10-21)18-17-5-3-2-4-14(17)8-11-22-16(13-23)12-20-19(18)22/h2-5,12-13H,6-11H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Alcaftadine Dilution Calculator

Alcaftadine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2532 mL | 16.266 mL | 32.532 mL | 65.0639 mL | 81.3299 mL |
| 5 mM | 0.6506 mL | 3.2532 mL | 6.5064 mL | 13.0128 mL | 16.266 mL |
| 10 mM | 0.3253 mL | 1.6266 mL | 3.2532 mL | 6.5064 mL | 8.133 mL |
| 50 mM | 0.0651 mL | 0.3253 mL | 0.6506 mL | 1.3013 mL | 1.6266 mL |
| 100 mM | 0.0325 mL | 0.1627 mL | 0.3253 mL | 0.6506 mL | 0.8133 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Alcaftadine(R89674) is a H1 histamine receptor antagonist, which is used to prevent eye irritation brought on by allergic conjunctivitis. Target: H1 Histamine Receptor Alcaftadine is a broad-spectrum antihistamine displaying a high affinity for histamine H1 and H2 receptors and a lower affinity for H4 receptors. alcaftadine was more effective than placebo and at least as effective as olopatadine 0.01% in preventing ocular itching at 15 minutes and at 16 hours after administration. Alcaftadine 0.025% ophthalmic solution has been approved by the U.S. Food and Drug Administration for prevention of itching associated with allergic conjunctivitis in patients over 2 years of age [1]. Alcaftadine is a safe and effective option for the prevention of ocular itching associated with allergic conjunctivitis, is dosed once daily, and is competitively priced among prescription medications for allergic conjunctivitis [2].
References:
[1]. Namdar, R. and C. Valdez, Alcaftadine: a topical antihistamine for use in allergic conjunctivitis. Drugs Today (Barc), 2011. 47(12): p. 883-90.
[2]. Mahvan, T.D., W.A. Buckley, and J.R. Hornecker, Alcaftadine for the prevention of itching associated with allergic conjunctivitis. Ann Pharmacother, 2012. 46(7-8): p. 1025-32.
- Trovafloxacin mesylate
Catalog No.:BCC3931
CAS No.:147059-75-4
- Rocaglaol
Catalog No.:BCN1653
CAS No.:147059-46-9
- Cyclo(Phe-Pro)
Catalog No.:BCN2416
CAS No.:14705-60-3
- MK591
Catalog No.:BCC1766
CAS No.:147030-01-1
- Menthyl-5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-[1,3]oxathiolane-2-carboxylic acid
Catalog No.:BCC9019
CAS No.:147027-10-9
- 3'-O-Demethylarctigenin
Catalog No.:BCN3544
CAS No.:147022-95-5
- Cytarabine
Catalog No.:BCC3759
CAS No.:147-94-4
- Proline
Catalog No.:BCN1656
CAS No.:147-85-3
- DL-Arabinose
Catalog No.:BCN8541
CAS No.:147-81-9
- Diphenhydramine hydrochloride
Catalog No.:BCC8947
CAS No.:147-24-0
- QL47
Catalog No.:BCC3920
CAS No.:1469988-75-7
- Atglistatin
Catalog No.:BCC5104
CAS No.:1469924-27-3
- 5,6-Dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-4-one
Catalog No.:BCC8722
CAS No.:147086-79-1
- Rosuvastatin Calcium
Catalog No.:BCC3853
CAS No.:147098-20-2
- Maropitant
Catalog No.:BCC1728
CAS No.:147116-67-4
- Methyl (3R)-3-(tert-butyldimethylsilyloxy)-5-oxo-6-triphenylphosphoranylidenehexanoate
Catalog No.:BCC9031
CAS No.:147118-35-2
- 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidinyl-5-yl-formyl
Catalog No.:BCC8651
CAS No.:147118-37-4
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- 4-Chloro-L-phenylalanine Hydrochloride
Catalog No.:BCC2638
CAS No.:123053-23-6
- TT 232
Catalog No.:BCC6248
CAS No.:147159-51-1
- N-6-Methyl-7,7-dioxo-2-sulfamoyl-5,6-dihydro-4H-thieno[2,3-b]thiopyran-4-yl]acetamide
Catalog No.:BCC9077
CAS No.:147200-03-1
- Arecaidine propargyl ester tosylate
Catalog No.:BCC6628
CAS No.:147202-94-6
- Delavirdine mesylate
Catalog No.:BCC4069
CAS No.:147221-93-0
- glatiramer acetate
Catalog No.:BCC5642
CAS No.:147245-92-9
Update and clinical utility of alcaftadine ophthalmic solution 0.25% in the treatment of allergic conjunctivitis.[Pubmed:26185412]
Clin Ophthalmol. 2015 Jul 8;9:1215-25.
Allergic disorders of the ocular surface are primarily characterized as IgE- and/or T-lymphocyte-mediated disorders that affect the cornea, conjunctiva, and eyelid. Approximately 40% of individuals in the developed countries have allergic conjunctivitis, and as such, it is the most common form of ocular allergy. Seasonal allergic conjunctivitis is the most prevalent type of allergic conjunctivitis that impacts the quality of life of patients. This article reviews the pharmacology, pharmacodynamics, pharmacokinetics, clinical trials, clinical efficacy, and safety of Alcaftadine. Histamine and the pathological mechanism of ocular allergy will be briefly reviewed with the intent of providing a background for the detailed discussion on the clinical utility of Alcaftadine in allergic conjunctivitis. The Medline PubMed, Elsevier Science Direct, and Google Scholar databases were used to search for evidence-based literature on histamine and immunopathological mechanism of allergic conjunctivitis, as well as on pharmacology, pharmacodynamics, pharmacokinetics, clinical trials, and clinical efficacy of Alcaftadine. The treatment and management goals of allergic conjunctivitis are to prevent or minimize the inflammatory cascade associated with allergic response in the early stages of the pathological mechanism. It is of note that activation of histamine receptors on immune and nonimmune cells are associated with allergen-induced inflammation of the conjunctiva and its associated ocular allergic manifestations, including itching, edema, hyperemia, and tearing. Alcaftadine is an efficacious multiple action antiallergic therapeutic agent with inverse agonist activity on H1, H2, and H4 receptors, as well as anti-inflammatory and mast cell stabilizing effects that could provide therapeutic benefits to patients with allergic conjunctivitis.
Synthesis and structural elucidation of a novel polymorph of alcaftadine.[Pubmed:25706601]
Spectrochim Acta A Mol Biomol Spectrosc. 2015 May 5;142:311-9.
In this study, we have synthesized and elucidated the structure of the H1 histamine antagonist, 2-(1-methylpiperidin-4-ylidene)-4,7-diazatricyclo[8.4.0.0((3,7))]tetradeca-1(14), 3,5,10,12-pentaene-6-carbaldehyde in the solution and solid-state. We have also studied the thermal dilapidation of the compound. Solution structure analysis was achieved by employing NMR spectroscopy including 2D experiments NOESY, HSQC and HMBC, while solid state investigations were undertaken using SXRD, PXRD, TGA, DSC, and IR spectroscopy. For the first time the single crystal structure of Alcaftadine has now been solved. Crystallographic data are as follows: monoclinic, Cc, a=11.5694(6)A, b=14.5864(6)A, c=10.2688(4)A, alpha=90 degrees , beta=111.793(3) degrees , gamma=90 degrees , V=1609.07(13)A(3), Z=4. The Hirshfeld surface analyses also have been performed using the crystal structure.
Effect of alcaftadine 0.25% on ocular itch associated with seasonal or perennial allergic conjunctivitis: a pooled analysis of two multicenter randomized clinical trials.[Pubmed:25999684]
Clin Ophthalmol. 2015 May 2;9:765-72.
PURPOSE: Seasonal and perennial allergic conjunctivitis represent the majority of cases of ocular allergy. This analysis was designed to evaluate the efficacy and safety of once-daily Alcaftadine 0.25% in preventing ocular itching associated with seasonal or perennial allergic conjunctivitis. SUBJECTS AND METHODS: Pooled data from two double-masked, multicenter, placebo-controlled studies using the conjunctival allergen challenge (CAC) model of allergic conjunctivitis were analyzed. Subjects randomized to receive treatment with Alcaftadine 0.25% or placebo were challenged with seasonal (grass, ragweed, trees) or perennial (cat dander, cat hair, dog dander, dust mites, cockroach) allergens, 16 hours after treatment instillation. The primary efficacy measure was subject-evaluated mean ocular itching at 3 minutes post-CAC. Secondary measures included ocular itching at 5 and 7 minutes post-CAC. The proportion of subjects with minimal itch (itch score <1) and zero itch (itch score =0), and safety were also assessed. RESULTS: A total of 189 subjects enrolled in the two studies were treated with Alcaftadine or placebo. Overall, 129 subjects were challenged with seasonal allergens and 60 subjects were challenged with perennial allergens. Alcaftadine 0.25% achieved a statistically significant reduction in mean itch score at 3, 5, and 7 minutes post-CAC compared with placebo in subjects challenged with seasonal allergens (P<0.0001 at all time points) and those challenged with perennial allergens (P<0.0001 at all time points). A higher percentage of subjects treated with Alcaftadine compared with placebo achieved minimal itch (PAlcaftadine 0.25% ophthalmic solution was well tolerated and demonstrated effective relief of ocular itching in subjects challenged with allergens classic for triggering either seasonal or perennial allergic conjunctivitis.
Ocular itch relief with alcaftadine 0.25% versus olopatadine 0.2% in allergic conjunctivitis: pooled analysis of two multicenter randomized clinical trials.[Pubmed:25260889]
Adv Ther. 2014 Oct;31(10):1059-71.
INTRODUCTION: The efficacy and safety of the once-daily topical ophthalmic solutions, Alcaftadine 0.25% and olopatadine 0.2%, in preventing ocular itching associated with allergic conjunctivitis were evaluated. METHODS: Pooled analysis was conducted of two double-masked, multicenter, active- and placebo-controlled studies using the conjunctival allergen challenge (CAC) model of allergic conjunctivitis. Subjects were randomized 1:1:1 to receive Alcaftadine 0.25%, olopatadine 0.2%, or placebo. The primary efficacy measure was subject-evaluated mean ocular itching at 3 min post-CAC and 16 h after treatment instillation. Secondary measures included ocular itching at 5 and 7 min post-CAC. Ocular itch was determined over all time points measured (3, 5, and 7 min) post-CAC and the proportion of subjects with minimal itch (itch score<1) and zero itch (itch score=0) was also assessed. RESULTS: A total of 284 subjects were enrolled in the two studies. At 3 min post-CAC and 16 h after treatment instillation, Alcaftadine 0.25% achieved a significantly lower mean itch score compared with olopatadine 0.2% (0.50 vs. 0.87, respectively; P=0.0006). Alcaftadine demonstrated a significantly lower mean itch score over all time points compared with olopatadine (0.68 vs. 0.92, respectively; P=0.0390); both Alcaftadine- and olopatadine-treated subjects achieved significantly lower overall mean ocular itching scores compared with placebo (2.10; P<0.0001 for both actives). Minimal itch over all time points was reported by 76.1% of Alcaftadine-treated subjects compared with 58.1% of olopatadine-treated subjects (P=0.0121). Treatment with Alcaftadine 0.25% and olopatadine 0.2% was safe and well tolerated; no serious adverse events were reported. CONCLUSION: Once-daily Alcaftadine 0.25% ophthalmic solution demonstrated greater efficacy in prevention of ocular itching compared with olopatadine 0.2% at 3 min post-CAC (primary endpoint), and over all time points, 16 h post-treatment instillation. Alcaftadine and olopatadine both provided effective relief compared with placebo and were generally well tolerated.


