WZ4003NUAK1/2 inhibitor, potent and selective CAS# 1214265-58-3 |
- Dacomitinib (PF299804, PF299)
Catalog No.:BCC3683
CAS No.:1110813-31-4
- AG-1478
Catalog No.:BCC3717
CAS No.:153436-53-4
- OSI-420
Catalog No.:BCC4472
CAS No.:183320-51-6
- Gefitinib hydrochloride
Catalog No.:BCC1591
CAS No.:184475-55-6
- Lapatinib Ditosylate
Catalog No.:BCC2083
CAS No.:388082-78-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1214265-58-3 | SDF | Download SDF |
| PubChem ID | 72200024 | Appearance | Powder |
| Formula | C25H29ClN6O3 | M.Wt | 496.99 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 33.33 mg/mL (67.06 mM; Need ultrasonic) | ||
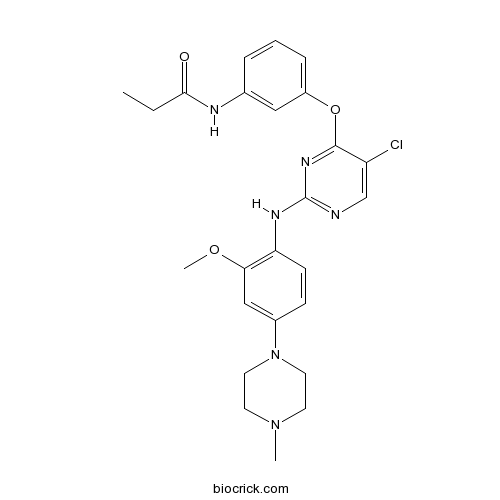
| Chemical Name | N-[3-[5-chloro-2-[2-methoxy-4-(4-methylpiperazin-1-yl)anilino]pyrimidin-4-yl]oxyphenyl]propanamide | ||
| SMILES | CCC(=O)NC1=CC(=CC=C1)OC2=NC(=NC=C2Cl)NC3=C(C=C(C=C3)N4CCN(CC4)C)OC | ||
| Standard InChIKey | SDGJBAUIGHSMRI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H29ClN6O3/c1-4-23(33)28-17-6-5-7-19(14-17)35-24-20(26)16-27-25(30-24)29-21-9-8-18(15-22(21)34-3)32-12-10-31(2)11-13-32/h5-9,14-16H,4,10-13H2,1-3H3,(H,28,33)(H,27,29,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective NUAK1/2 inhibitor (IC50 values are 20 and 100 nM respectively). Exhibits no significant inhibition against a panel of 139 kinases, including ten AMPK family members. Inhibits NUAK1-mediated MYPT1 phosphorylation. Also inhibits cell proliferation in U2OS cells. |

WZ4003 Dilution Calculator

WZ4003 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0121 mL | 10.0606 mL | 20.1211 mL | 40.2423 mL | 50.3028 mL |
| 5 mM | 0.4024 mL | 2.0121 mL | 4.0242 mL | 8.0485 mL | 10.0606 mL |
| 10 mM | 0.2012 mL | 1.0061 mL | 2.0121 mL | 4.0242 mL | 5.0303 mL |
| 50 mM | 0.0402 mL | 0.2012 mL | 0.4024 mL | 0.8048 mL | 1.0061 mL |
| 100 mM | 0.0201 mL | 0.1006 mL | 0.2012 mL | 0.4024 mL | 0.503 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
WZ4003 is a potent and selective inhibitor of NUAK1 and NUAK2 with IC50 values of 20 and 100 nM, respectively [1].
NUAK family SNF1-like kinase-1 (NUAK1) and the related NUAK2 belong to the AMP-activated protein kinase (AMPK) family and are activated by liver kinase B1 (LKB1) tumour suppressor protein kinase [1].
WZ4003 is a potent and selective NUAK1/2 inhibitor. In HEK-293 cells, WZ4003 inhibited the phosphorylation of myosin phosphate-targeting subunit 1 (MYPT1), which was phosphorylated by NUAK1 at Ser445. In HEK-293 cells overexpressing inhibitor-resistant NUAK1[A195T], WZ4003 didn’t inhibit the phosphorylation of MYPT1 at Ser445. In mouse embryonic fibroblasts (MEFs), WZ4003 significantly inhibited migration in a wound-healing assay and inhibited MEFs proliferation. In a cell invasion assay, WZ4003 inhibited the invasive potential of U2OS cells [1]. In U2OS cells, WZ4003 (10 μM) inhibited phosphorylation of MYPT1 and reduced the cells in S-phase by 50%. Also, WZ4003 prevented cells from entering into mitosis [2].
References:
[1]. Banerjee S, Buhrlage SJ, Huang HT, et al. Characterization of WZ4003 and HTH-01-015 as selective inhibitors of the LKB1-tumour-suppressor-activated NUAK kinases. Biochem J, 2014, 457(1): 215-225.
[2]. Banerjee S, Zagórska A, Deak M, et al. Interplay between Polo kinase, LKB1-activated NUAK1 kinase, PP1βMYPT1 phosphatase complex and the SCFβTrCP E3 ubiquitin ligase. Biochem J, 2014, 461(2): 233-245.
- WZ8040
Catalog No.:BCC1075
CAS No.:1214265-57-2
- WZ3146
Catalog No.:BCC4004
CAS No.:1214265-56-1
- POM 1
Catalog No.:BCC7454
CAS No.:12141-67-2
- N6-Benzyladenine
Catalog No.:BCC9076
CAS No.:1214-39-7
- Bernardioside A
Catalog No.:BCN7862
CAS No.:121368-52-3
- SR 33805 oxalate
Catalog No.:BCC7181
CAS No.:121346-33-6
- Fmoc-Glu-OH
Catalog No.:BCC3489
CAS No.:121343-82-6
- WZ4002
Catalog No.:BCC1074
CAS No.:1213269-23-8
- 1-Hydroxybisabola-2,10-dien-4-one
Catalog No.:BCN7297
CAS No.:1213251-45-6
- RWJ 21757
Catalog No.:BCC7460
CAS No.:121288-39-9
- Alendronate
Catalog No.:BCC4885
CAS No.:121268-17-5
- ICI 204,448 hydrochloride
Catalog No.:BCC6806
CAS No.:121264-04-8
- Ajugapantin A
Catalog No.:BCN3663
CAS No.:121449-67-0
- Daclatasvir (BMS-790052)
Catalog No.:BCC2533
CAS No.:1214735-16-6
- 5-Benzyloxyindole
Catalog No.:BCC8742
CAS No.:1215-59-4
- PF-04991532
Catalog No.:BCC8094
CAS No.:1215197-37-7
- Salvianolic acid B; Lithospermic acid B; Danfensuan B
Catalog No.:BCC8249
CAS No.:121521-90-2
- SR 58611A hydrochloride
Catalog No.:BCC7833
CAS No.:121524-09-2
- GR 144053 trihydrochloride
Catalog No.:BCC6998
CAS No.:1215333-48-4
- RG2833
Catalog No.:BCC1893
CAS No.:1215493-56-3
- CFM 1571 hydrochloride
Catalog No.:BCC5924
CAS No.:1215548-30-3
- SB 242084
Catalog No.:BCC5949
CAS No.:1215566-78-1
- RS 100329 hydrochloride
Catalog No.:BCC5741
CAS No.:1215654-26-4
- CP-809101 hydrochloride
Catalog No.:BCC1499
CAS No.:1215721-40-6
Characterization of WZ4003 and HTH-01-015 as selective inhibitors of the LKB1-tumour-suppressor-activated NUAK kinases.[Pubmed:24171924]
Biochem J. 2014 Jan 1;457(1):215-25.
The related NUAK1 and NUAK2 are members of the AMPK (AMP-activated protein kinase) family of protein kinases that are activated by the LKB1 (liver kinase B1) tumour suppressor kinase. Recent work suggests they play important roles in regulating key biological processes including Myc-driven tumorigenesis, senescence, cell adhesion and neuronal polarity. In the present paper we describe the first highly specific protein kinase inhibitors of NUAK kinases namely WZ4003 and HTH-01-015. WZ4003 inhibits both NUAK isoforms (IC50 for NUAK1 is 20 nM and for NUAK2 is 100 nM), whereas HTH-01-015 inhibits only NUAK1 (IC50 is 100 nM). These compounds display extreme selectivity and do not significantly inhibit the activity of 139 other kinases that were tested including ten AMPK family members. In all cell lines tested, WZ4003 and HTH-01-015 inhibit the phosphorylation of the only well-characterized substrate, MYPT1 (myosin phosphate-targeting subunit 1) that is phosphorylated by NUAK1 at Ser(445). We also identify a mutation (A195T) that does not affect basal NUAK1 activity, but renders it ~50-fold resistant to both WZ4003 and HTH-01-015. Consistent with NUAK1 mediating the phosphorylation of MYPT1 we find that in cells overexpressing drug-resistant NUAK1[A195T], but not wild-type NUAK1, phosphorylation of MYPT1 at Ser(445) is no longer suppressed by WZ4003 or HTH-01-015. We also demonstrate that administration of WZ4003 and HTH-01-015 to MEFs (mouse embryonic fibroblasts) significantly inhibits migration in a wound-healing assay to a similar extent as NUAK1-knockout. WZ4003 and HTH-01-015 also inhibit proliferation of MEFs to the same extent as NUAK1 knockout and U2OS cells to the same extent as NUAK1 shRNA knockdown. We find that WZ4003 and HTH-01-015 impaired the invasive potential of U2OS cells in a 3D cell invasion assay to the same extent as NUAK1 knockdown. The results of the present study indicate that WZ4003 and HTH-01-015 will serve as useful chemical probes to delineate the biological roles of the NUAK kinases.
Interplay between Polo kinase, LKB1-activated NUAK1 kinase, PP1betaMYPT1 phosphatase complex and the SCFbetaTrCP E3 ubiquitin ligase.[Pubmed:24785407]
Biochem J. 2014 Jul 15;461(2):233-45.
NUAK1 (NUAK family SnF1-like kinase-1) and NUAK2 protein kinases are activated by the LKB1 tumour suppressor and have been implicated in regulating multiple processes such as cell survival, senescence, adhesion and polarity. In the present paper we present evidence that expression of NUAK1 is controlled by CDK (cyclin-dependent kinase), PLK (Polo kinase) and the SCFbetaTrCP (Skp, Cullin and F-boxbetaTrCP) E3 ubiquitin ligase complex. Our data indicate that CDK phosphorylates NUAK1 at Ser445, triggering binding to PLK, which subsequently phosphorylates NUAK1 at two conserved non-catalytic serine residues (Ser476 and Ser480). This induces binding of NUAK1 to betaTrCP, the substrate-recognition subunit of the SCFbetaTrCP E3 ligase, resulting in NUAK1 becoming ubiquitylated and degraded. We also show that NUAK1 and PLK1 are reciprocally controlled in the cell cycle. In G2-M-phase, when PLK1 is most active, NUAK1 levels are low and vice versa in S-phase, when PLK1 expression is low, NUAK1 is more highly expressed. Moreover, NUAK1 inhibitors (WZ4003 or HTH-01-015) suppress proliferation by reducing the population of cells in S-phase and mitosis, an effect that can be rescued by overexpression of a NUAK1 mutant in which Ser476 and Ser480 are mutated to alanine. Finally, previous work has suggested that NUAK1 phosphorylates and inhibits PP1betaMYPT1 (where PP1 is protein phosphatase 1) and that a major role for the PP1betaMYPT1 complex is to inhibit PLK1 by dephosphorylating its T-loop (Thr210). We demonstrate that activation of NUAK1 leads to a striking increase in phosphorylation of PLK1 at Thr210, an effect that is suppressed by NUAK1 inhibitors. Our data link NUAK1 to important cell-cycle signalling components (CDK, PLK and SCFbetaTrCP) and suggest that NUAK1 plays a role in stimulating S-phase, as well as PLK1 activity via its ability to regulate the PP1betaMYPT1 phosphatase.
Novel mutant-selective EGFR kinase inhibitors against EGFR T790M.[Pubmed:20033049]
Nature. 2009 Dec 24;462(7276):1070-4.
The clinical efficacy of epidermal growth factor receptor (EGFR) kinase inhibitors in EGFR-mutant non-small-cell lung cancer (NSCLC) is limited by the development of drug-resistance mutations, including the gatekeeper T790M mutation. Strategies targeting EGFR T790M with irreversible inhibitors have had limited success and are associated with toxicity due to concurrent inhibition of wild-type EGFR. All current EGFR inhibitors possess a structurally related quinazoline-based core scaffold and were identified as ATP-competitive inhibitors of wild-type EGFR. Here we identify a covalent pyrimidine EGFR inhibitor by screening an irreversible kinase inhibitor library specifically against EGFR T790M. These agents are 30- to 100-fold more potent against EGFR T790M, and up to 100-fold less potent against wild-type EGFR, than quinazoline-based EGFR inhibitors in vitro. They are also effective in murine models of lung cancer driven by EGFR T790M. Co-crystallization studies reveal a structural basis for the increased potency and mutant selectivity of these agents. These mutant-selective irreversible EGFR kinase inhibitors may be clinically more effective and better tolerated than quinazoline-based inhibitors. Our findings demonstrate that functional pharmacological screens against clinically important mutant kinases represent a powerful strategy to identify new classes of mutant-selective kinase inhibitors.


