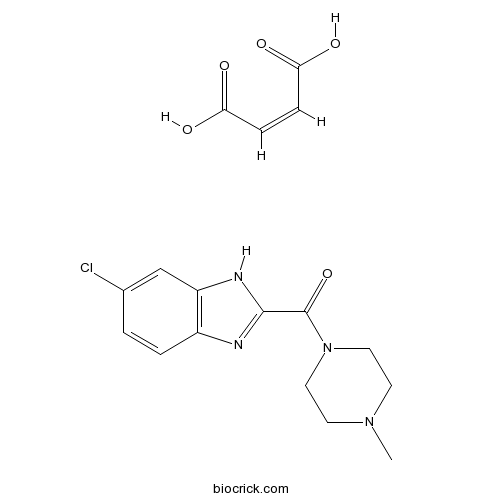
JNJ 10191584 maleateSelective H4 receptor antagonist; orally active CAS# 869497-75-6 |
- Cyclopamine
Catalog No.:BCN2964
CAS No.:4449-51-8
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- GANT61
Catalog No.:BCC1090
CAS No.:500579-04-4
- GANT 58
Catalog No.:BCC1587
CAS No.:64048-12-0
- GDC-0449 (Vismodegib)
Catalog No.:BCC1285
CAS No.:879085-55-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 869497-75-6 | SDF | Download SDF |
| PubChem ID | 11718163 | Appearance | Powder |
| Formula | C17H19ClN4O5 | M.Wt | 394.81 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | VUF 6002 | ||
| Solubility | Soluble to 50 mM in DMSO | ||
| Chemical Name | (Z)-but-2-enedioic acid;(6-chloro-1H-benzimidazol-2-yl)-(4-methylpiperazin-1-yl)methanone | ||
| SMILES | CN1CCN(CC1)C(=O)C2=NC3=C(N2)C=C(C=C3)Cl.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | KOTJFAYEELTYCZ-BTJKTKAUSA-N | ||
| Standard InChI | InChI=1S/C13H15ClN4O.C4H4O4/c1-17-4-6-18(7-5-17)13(19)12-15-10-3-2-9(14)8-11(10)16-12;5-3(6)1-2-4(7)8/h2-3,8H,4-7H2,1H3,(H,15,16);1-2H,(H,5,6)(H,7,8)/b;2-1- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly selective histamine H4 receptor silent antagonist; binds with high affinity to the human H4 receptor (Ki = 26 nM). > 540-fold selective over the H3 receptor (Ki = 14.1 μM). Inhibits mast cell and eosinophil chemotaxis in vitro with IC50 values of 138 and 530 nM respectively. Orally active in vivo. |

JNJ 10191584 maleate Dilution Calculator

JNJ 10191584 maleate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5329 mL | 12.6643 mL | 25.3286 mL | 50.6573 mL | 63.3216 mL |
| 5 mM | 0.5066 mL | 2.5329 mL | 5.0657 mL | 10.1315 mL | 12.6643 mL |
| 10 mM | 0.2533 mL | 1.2664 mL | 2.5329 mL | 5.0657 mL | 6.3322 mL |
| 50 mM | 0.0507 mL | 0.2533 mL | 0.5066 mL | 1.0131 mL | 1.2664 mL |
| 100 mM | 0.0253 mL | 0.1266 mL | 0.2533 mL | 0.5066 mL | 0.6332 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Praeroside II
Catalog No.:BCN7001
CAS No.:86940-46-7
- 14-Deoxy-17-hydroxyandrographolide
Catalog No.:BCN4560
CAS No.:869384-82-7
- MLN8054
Catalog No.:BCC2170
CAS No.:869363-13-3
- AZD8330
Catalog No.:BCC3733
CAS No.:869357-68-6
- Neurokinin B (human, porcine)
Catalog No.:BCC7119
CAS No.:86933-75-7
- Neurokinin A (porcine)
Catalog No.:BCC6955
CAS No.:86933-74-6
- PF-573228
Catalog No.:BCC4496
CAS No.:869288-64-2
- SB 203580 hydrochloride
Catalog No.:BCC4293
CAS No.:869185-85-3
- Eudesma-3,11-dien-2-one
Catalog No.:BCN7607
CAS No.:86917-79-5
- Umeclidinium bromide
Catalog No.:BCC2022
CAS No.:869113-09-7
- (R)-(-)-α-Methylhistamine dihydrobromide
Catalog No.:BCC5665
CAS No.:868698-49-1
- 3',5'-Dimethoxybiphenyl-3-ol
Catalog No.:BCN7529
CAS No.:868666-20-0
- Tecovirimat
Catalog No.:BCC5518
CAS No.:869572-92-9
- Fmoc-Tyr(tBu)-ol
Catalog No.:BCC2572
CAS No.:86967-51-3
- Obestatin (rat)
Catalog No.:BCC5912
CAS No.:869705-22-6
- A-770041
Catalog No.:BCC1323
CAS No.:869748-10-7
- threo-Guaiacylglycerol beta-coniferyl ether
Catalog No.:BCN1323
CAS No.:869799-76-8
- A 841720
Catalog No.:BCC7550
CAS No.:869802-58-4
- Andropanolide
Catalog No.:BCN4559
CAS No.:869807-57-8
- Formoxanthone A
Catalog No.:BCN6451
CAS No.:869880-32-0
- Radezolid
Catalog No.:BCC1882
CAS No.:869884-78-6
- VRT752271
Catalog No.:BCC4122
CAS No.:869886-67-9
- Alpinumisoflavone acetate
Catalog No.:BCN6813
CAS No.:86989-18-6
- MK-2048
Catalog No.:BCC2136
CAS No.:869901-69-9
Inhibitory effects of histamine H4 receptor antagonists on experimental colitis in the rat.[Pubmed:16213481]
Eur J Pharmacol. 2005 Oct 17;522(1-3):130-8.
The histamine H(4) receptor is a G-protein coupled receptor with little homology to the pro-inflammatory histamine H(1) receptor, expressed on cells of the immune system with hematopoietic lineage such as eosinophils and mast cells. The effects of the recently described highly selective histamine H(4) receptor antagonists JNJ 10191584 and JNJ 7777120 have now been investigated on the acute colitis provoked by trinitrobenzene sulphonic acid over 3 days in the rat. Treatment with JNJ 10191584 (10-100 mg/kg p.o., b.i.d.) caused a dose-dependent reduction in macroscopic damage, inhibition of the TNBS-provoked elevation of both colonic myeloperoxidase and tumour necrosis factor-alpha (TNF-alpha), and a reduction in the histologically assessed increase in mucosal and submucosal thickness and neutrophil infiltration. JNJ 7777120 (100 mg/kg p.o., b.i.d.) likewise reduced the macroscopic injury and the increases in colonic myeloperoxidase and TNF-alpha levels. These findings indicate a pro-inflammatory role for the histamine H(4) receptor in this model and suggest a novel pharmacological approach to the treatment of colitis.
Preparation and biological evaluation of indole, benzimidazole, and thienopyrrole piperazine carboxamides: potent human histamine h(4) antagonists.[Pubmed:16366610]
J Med Chem. 2005 Dec 29;48(26):8289-98.
Three series of H(4) receptor ligands, derived from indoly-2-yl-(4-methyl-piperazin-1-yl)-methanones, have been synthesized and their structure-activity relationships evaluated for activity at the H(4) receptor in competitive binding and functional assays. In all cases, substitution of small lipophilic groups in the 4 and 5-positions led to increased activity in a [(3)H]histamine radiolabeled ligand competitive binding assay. In vitro metabolism and initial pharmacokinetic studies were performed on selected compounds leading to the identification of indole 8 and benzimidazole 40 as potent H(4) antagonists with the potential for further development. In addition, both 8 and 40 demonstrated efficacy in in vitro mast cell and eosinophil chemotaxis assays.
Synthesis and structure-activity relationships of indole and benzimidazole piperazines as histamine H(4) receptor antagonists.[Pubmed:15454206]
Bioorg Med Chem Lett. 2004 Nov 1;14(21):5251-6.
We describe the structure-activity relationships for a series of ligands structurally related to the recently identified (5-chloro-1H-indol-2-yl)-(4-methyl-piperazin-1-yl)-methanone (1) as histamine H(4) receptor (H(4)R) antagonists. Furthermore, we identified related benzimidazoles as novel lead compounds for the H(4)R. The ligands have been evaluated by radioligand displacement studies and functional assays for their interaction with both the human histamine H(3) and H(4) receptors and exhibit pK(i) values up to 7.5 at the human H(4)R.


