SB 203580 hydrochlorideSpecific p38-MAPKs inhibitor CAS# 869185-85-3 |
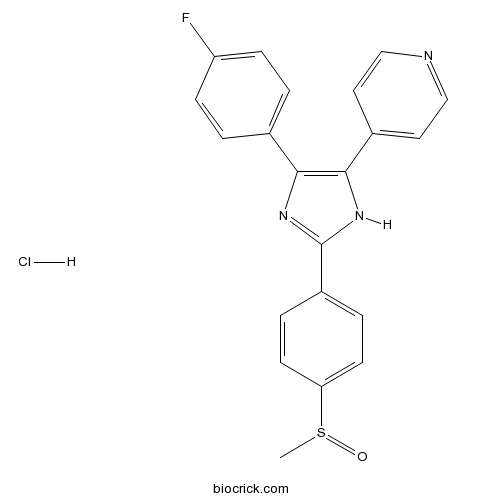
- SB 203580
Catalog No.:BCC3663
CAS No.:152121-47-6
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- PH-797804
Catalog No.:BCC3672
CAS No.:586379-66-0
- LY2228820
Catalog No.:BCC2528
CAS No.:862507-23-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 869185-85-3 | SDF | Download SDF |
| PubChem ID | 16760644 | Appearance | Powder |
| Formula | C21H17ClFN3OS | M.Wt | 413.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | RWJ 64809 hydrochloride | ||
| Solubility | DMSO : 100 mg/mL (241.60 mM; Need ultrasonic) H2O : 8.43 mg/mL (20.37 mM; Need ultrasonic and warming) | ||
| Chemical Name | 4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine;hydrochloride | ||
| SMILES | CS(=O)C1=CC=C(C=C1)C2=NC(=C(N2)C3=CC=NC=C3)C4=CC=C(C=C4)F.Cl | ||
| Standard InChIKey | WOSGGXINSLMASH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H16FN3OS.ClH/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15;/h2-13H,1H3,(H,24,25);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Water-soluble salt of SB 203580. Selective inhibitor of p38 mitogen-activated protein kinase (IC50 values are 50 and 500 nM for SAPK2a/p38 and SAPK2b/p38β2 respectively). Displays 100-500-fold selectivity over LCK, GSK3β and PKBα. Shown to inhibit interleukin-2-induced T cell proliferation, cyclooxygenase-1 and -2, and thromboxane synthase. |

SB 203580 hydrochloride Dilution Calculator

SB 203580 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.416 mL | 12.0802 mL | 24.1604 mL | 48.3209 mL | 60.4011 mL |
| 5 mM | 0.4832 mL | 2.416 mL | 4.8321 mL | 9.6642 mL | 12.0802 mL |
| 10 mM | 0.2416 mL | 1.208 mL | 2.416 mL | 4.8321 mL | 6.0401 mL |
| 50 mM | 0.0483 mL | 0.2416 mL | 0.4832 mL | 0.9664 mL | 1.208 mL |
| 100 mM | 0.0242 mL | 0.1208 mL | 0.2416 mL | 0.4832 mL | 0.604 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SB203580 HCl is a specific inhibitor of p38-MAPKs with IC50 value of 0.6 μM [1].
SB203580 is an inhibitor of p38-MAPKα and p38-MAPKβ. In neonatal myocytes, SB203580 prevented p38-MAPK from activating MAPKAPK2 with IC50 value of 70 nM. It also significantly suppressed MAPKAPK2 activation in by IL-1, osmotic stress or arsenite in KB cells. In neonatal rat ventricular myocytes, SB203580 inhibited JNK activity of activating c-Jun with IC50 value of 3-10 μM. Besides that, SB203580 affected the phosphorylation of small heat shock proteins caused by MAPKAPK2. It inhibited the IL-1-, chemical- or osmotic stress-stimulated HSP27 phosphorylation with IC50 value of < 1μM in KB cells [1, 2].
References:
[1] Cuenda A, Rouse J, Doza Y N, et al. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS letters, 1995, 364(2): 229-233.
[2] Clerk A, Sugden P H. The p38-MAPK inhibitor, SB203580, inhibits cardiac stress-activated protein kinases/c-Jun N-terminal kinases (SAPKs/JNKs). FEBS letters, 1998, 426(1): 93-96.
- Eudesma-3,11-dien-2-one
Catalog No.:BCN7607
CAS No.:86917-79-5
- Umeclidinium bromide
Catalog No.:BCC2022
CAS No.:869113-09-7
- (R)-(-)-α-Methylhistamine dihydrobromide
Catalog No.:BCC5665
CAS No.:868698-49-1
- 3',5'-Dimethoxybiphenyl-3-ol
Catalog No.:BCN7529
CAS No.:868666-20-0
- Carfilzomib (PR-171)
Catalog No.:BCC1145
CAS No.:868540-17-4
- Iristectorigenin B
Catalog No.:BCN8391
CAS No.:39012-01-6
- (+)-Puerol B 2''-O-glucoside
Catalog No.:BCN4561
CAS No.:868409-19-2
- Protosappanin A dimethyl acetal
Catalog No.:BCN6517
CAS No.:868405-37-2
- 2,2,5,5-Tetramethylcyclohexane-1,4-dione
Catalog No.:BCN1324
CAS No.:86838-54-2
- WAY 213613
Catalog No.:BCC7442
CAS No.:868359-05-1
- Rhodiosin; Herbacetin-7-O-glucorhamnoside
Catalog No.:BCN8478
CAS No.:86831-54-1
- Rhodiolin
Catalog No.:BCC8356
CAS No.:86831-53-0
- PF-573228
Catalog No.:BCC4496
CAS No.:869288-64-2
- Neurokinin A (porcine)
Catalog No.:BCC6955
CAS No.:86933-74-6
- Neurokinin B (human, porcine)
Catalog No.:BCC7119
CAS No.:86933-75-7
- AZD8330
Catalog No.:BCC3733
CAS No.:869357-68-6
- MLN8054
Catalog No.:BCC2170
CAS No.:869363-13-3
- 14-Deoxy-17-hydroxyandrographolide
Catalog No.:BCN4560
CAS No.:869384-82-7
- Praeroside II
Catalog No.:BCN7001
CAS No.:86940-46-7
- JNJ 10191584 maleate
Catalog No.:BCC7362
CAS No.:869497-75-6
- Tecovirimat
Catalog No.:BCC5518
CAS No.:869572-92-9
- Fmoc-Tyr(tBu)-ol
Catalog No.:BCC2572
CAS No.:86967-51-3
- Obestatin (rat)
Catalog No.:BCC5912
CAS No.:869705-22-6
- A-770041
Catalog No.:BCC1323
CAS No.:869748-10-7
Specificity and mechanism of action of some commonly used protein kinase inhibitors.[Pubmed:10998351]
Biochem J. 2000 Oct 1;351(Pt 1):95-105.
The specificities of 28 commercially available compounds reported to be relatively selective inhibitors of particular serine/threonine-specific protein kinases have been examined against a large panel of protein kinases. The compounds KT 5720, Rottlerin and quercetin were found to inhibit many protein kinases, sometimes much more potently than their presumed targets, and conclusions drawn from their use in cell-based experiments are likely to be erroneous. Ro 318220 and related bisindoylmaleimides, as well as H89, HA1077 and Y 27632, were more selective inhibitors, but still inhibited two or more protein kinases with similar potency. LY 294002 was found to inhibit casein kinase-2 with similar potency to phosphoinositide (phosphatidylinositol) 3-kinase. The compounds with the most impressive selectivity profiles were KN62, PD 98059, U0126, PD 184352, rapamycin, wortmannin, SB 203580 and SB 202190. U0126 and PD 184352, like PD 98059, were found to block the mitogen-activated protein kinase (MAPK) cascade in cell-based assays by preventing the activation of MAPK kinase (MKK1), and not by inhibiting MKK1 activity directly. Apart from rapamycin and PD 184352, even the most selective inhibitors affected at least one additional protein kinase. Our results demonstrate that the specificities of protein kinase inhibitors cannot be assessed simply by studying their effect on kinases that are closely related in primary structure. We propose guidelines for the use of protein kinase inhibitors in cell-based assays.
Direct inhibition of cyclooxygenase-1 and -2 by the kinase inhibitors SB 203580 and PD 98059. SB 203580 also inhibits thromboxane synthase.[Pubmed:9786874]
J Biol Chem. 1998 Oct 30;273(44):28766-72.
The kinase inhibitors SB 203580 and PD 98059 have been reported to be specific inhibitors of the 38- and 42/44-kDa mitogen-activated protein kinase (MAPK) pathways, respectively. In this study, the two inhibitors were found to decrease platelet aggregation induced by low concentrations of arachidonic acid, suggesting that they also interfere with the metabolism of arachidonic acid to thromboxane A2. In support of this, SB 203580 and PD 98059 inhibited the conversion of exogenous [3H]arachidonic acid to [3H]thromboxane in intact platelets. Measurement of platelet cyclooxygenase-1 activity following immunoprecipitation revealed that SB 203580 and PD 98059 are direct inhibitors of this enzyme. Both compounds were shown to inhibit purified cyclooxygenase-1 and -2 by a reversible mechanism. In addition, SB 203580 (but not PD 98059) inhibited platelet aggregation induced by prostaglandin H2 and the conversion of prostaglandin H2 to thromboxane A2 in intact platelets. SB 203580 also inhibited this pathway in platelet microsome preparations, suggesting a direct inhibitory effect on thromboxane synthase. These results demonstrate that direct effects of the two kinase inhibitors on active arachidonic acid metabolites have to be excluded before using these compounds for the investigation of MAPKs in signal transduction pathways. This is of particular relevance to studies on the regulation of cytosolic phospholipase A2 as these two MAPKs are capable of phosphorylating cytosolic phospholipase A2, thereby increasing its intrinsic activity.
Role for p38 mitogen-activated protein kinase in platelet aggregation caused by collagen or a thromboxane analogue.[Pubmed:8636072]
J Biol Chem. 1996 Mar 22;271(12):6586-9.
p38 mitogen-activated protein kinase (MAPK) was identified in platelets on the basis of (a) its reactivity with antibodies to C-terminal and N-terminal peptides, and (b) its ability to activate MAPK-activated protein kinase-2, which phosphorylates the small heat shock protein, hsp27. p38 MAPK was activated in platelets by collagen fibers, a collagen-related cross-linked peptide, thrombin, or the thromboxane analogue U46619. A highly specific inhibitor of p38 MAPK, a pyridinyl imidazole known as SB203580, inhibited the platelet enzyme in vitro (IC50 approximately 0.5 microM). At similar concentrations it also inhibited agonist-stimulated phosphorylation of hsp27 in platelets, and platelet aggregation and secretion induced by minimal aggregatory concentrations of collagen or U46619, but not thrombin. Inhibition of aggregation was overcome by increasing agonist dose. SB203580 might act by inhibiting thromboxane generation, but this was only inhibited by 10-20% at low agonist concentrations. p38 MAPK provides a crucial signal, which is necessary for aggregation caused by minimal concentrations of collagen fibers or U46619. Thrombin or high doses of these agonists generate signals that bypass the enzyme, or render the enzyme no longer rate-limiting.
SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1.[Pubmed:7750577]
FEBS Lett. 1995 May 8;364(2):229-33.
A class of pyridinyl imidazoles inhibit the MAP kinase homologue, termed here reactivating kinase (RK) [Lee et al. (1994) Nature 372, 739-746]. We now show that one of these compounds (SB 203580) inhibits RK in vitro (IC50 = 0.6 microM), suppresses the activation of MAPKAP kinase-2 and prevents the phosphorylation of heat shock protein (HSP) 27 in response to interleukin-1, cellular stresses and bacterial endotoxin in vivo. These results establish that MAPKAP kinase-2 is a physiological RK substrate, and that HSP27 is phosphorylated by MAPKAP kinase-2 in vivo. The specificity of SB 203580 was indicated by its failure to inhibit 12 other protein kinases in vitro, and by its lack of effect on the activation of RK kinase and other MAP kinase cascades in vivo. We suggest that SB 203580 will be useful for identifying other physiological roles and targets of RK and MAPKAP kinase-2.


