L-KynurenineTryptophan catabolite; endogenous aryl hydrocarbon receptor ligand CAS# 2922-83-0 |
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
- LY341495
Catalog No.:BCC1724
CAS No.:201943-63-7
- MPEP Hydrochloride
Catalog No.:BCC1777
CAS No.:219911-35-0
- CPPHA
Catalog No.:BCC1501
CAS No.:693288-97-0
- Dipraglurant
Catalog No.:BCC1531
CAS No.:872363-17-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2922-83-0 | SDF | Download SDF |
| PubChem ID | 161166 | Appearance | Powder |
| Formula | C10H12N2O3 | M.Wt | 208.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (240.14 mM; Need ultrasonic) | ||
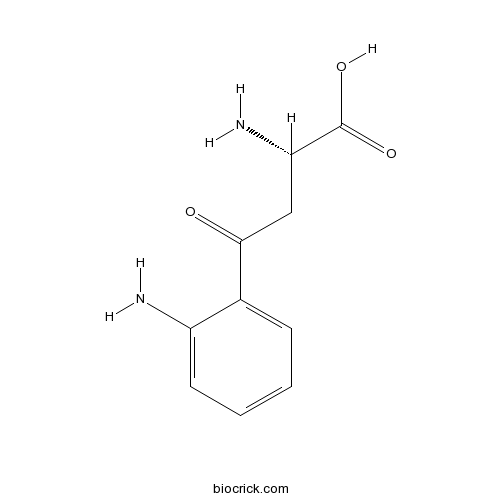
| Chemical Name | (2S)-2-amino-4-(2-aminophenyl)-4-oxobutanoic acid | ||
| SMILES | C1=CC=C(C(=C1)C(=O)CC(C(=O)O)N)N | ||
| Standard InChIKey | YGPSJZOEDVAXAB-QMMMGPOBSA-N | ||
| Standard InChI | InChI=1S/C10H12N2O3/c11-7-4-2-1-3-6(7)9(13)5-8(12)10(14)15/h1-4,8H,5,11-12H2,(H,14,15)/t8-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tryptophan catabolite; endogenous activator of the aryl hydrocarbon receptor (AhR). Immunosuppressant; suppresses allogeneic T cell proliferation. |

L-Kynurenine Dilution Calculator

L-Kynurenine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8028 mL | 24.0142 mL | 48.0284 mL | 96.0569 mL | 120.0711 mL |
| 5 mM | 0.9606 mL | 4.8028 mL | 9.6057 mL | 19.2114 mL | 24.0142 mL |
| 10 mM | 0.4803 mL | 2.4014 mL | 4.8028 mL | 9.6057 mL | 12.0071 mL |
| 50 mM | 0.0961 mL | 0.4803 mL | 0.9606 mL | 1.9211 mL | 2.4014 mL |
| 100 mM | 0.048 mL | 0.2401 mL | 0.4803 mL | 0.9606 mL | 1.2007 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tryptophan catabolite; endogenous activator of the aryl hydrocarbon receptor (AhR). Immunosuppressant; suppresses allogeneic T-cell proliferation.
- H-DL-Phe(4-NO2)-OH
Catalog No.:BCC3279
CAS No.:2922-40-9
- Adenine HCl
Catalog No.:BCC4453
CAS No.:2922-28-3
- Methylprednisolone hemisuccinate
Catalog No.:BCC9044
CAS No.:2921-57-5
- 3',6'-Bis(diethylamino)-2-(4-nitrophenyl)spiro[isoindole-1,9'-xanthene]-3-one
Catalog No.:BCC8597
CAS No.:29199-09-5
- Shegansu B
Catalog No.:BCN3381
CAS No.:291535-65-4
- [Ala11,22,28]VIP
Catalog No.:BCC5754
CAS No.:291524-04-4
- 1-Methoxyberberine
Catalog No.:BCN7373
CAS No.:29133-52-6
- (+)-Affinisine
Catalog No.:BCN3520
CAS No.:2912-11-0
- Guanfacine hydrochloride
Catalog No.:BCC1609
CAS No.:29110-48-3
- Guanfacine
Catalog No.:BCC5180
CAS No.:29110-47-2
- Procyanidin B2
Catalog No.:BCN6315
CAS No.:29106-49-8
- Pinoresinol dimethyl ether
Catalog No.:BCN6767
CAS No.:29106-36-3
- Dehydrotrametenolic acid
Catalog No.:BCN2718
CAS No.:29220-16-4
- SB-3CT
Catalog No.:BCC5486
CAS No.:292605-14-2
- 25,26-Dihydroxyvitamin D3
Catalog No.:BCC4201
CAS No.:29261-12-9
- L-685,458
Catalog No.:BCC2344
CAS No.:292632-98-5
- Deoxyelephantopin
Catalog No.:BCN4655
CAS No.:29307-03-7
- Genipin-1-O-gentiobioside
Catalog No.:BCN5349
CAS No.:29307-60-6
- Licarbazepine
Catalog No.:BCC7794
CAS No.:29331-92-8
- Tetrahydropalmatine
Catalog No.:BCN6310
CAS No.:2934-97-6
- Ciclopirox
Catalog No.:BCC4899
CAS No.:29342-05-0
- Olean-12-ene-3,11-dione
Catalog No.:BCN5195
CAS No.:2935-32-2
- H-Phg-OH
Catalog No.:BCC3310
CAS No.:2935-35-5
- SR3335
Catalog No.:BCC1964
CAS No.:293753-05-6
Serum concentrations of l-kynurenine predict clinical outcomes of patients with peripheral T-cell lymphoma, not otherwise specified.[Pubmed:27338762]
Hematol Oncol. 2017 Dec;35(4):637-644.
Indoleamine 2,3-dioxygenase exerts intense immunomodulatory effects due to enzymatic activities that catalyze the breakdown of the essential amino acid l-tryptophan. The activity of indoleamine 2,3-dioxygenase can be estimated by measuring serum L-Kynurenine concentrations. Here, we aimed to determine the role of L-Kynurenine as a prognostic factor for peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS) in a retrospective analysis of data derived from 31 consecutive patients between June 2000 and March 2013 who were histologically diagnosed with PTCL-NOS according to the World Health Organization classification and treated with 6-8 cycles of cyclophosphamide, doxorubicin or pirarubicin, vincristine, and prednisolone. L-Kynurenine concentrations in serum samples collected at admission were measured using high-performance liquid chromatography. The median serum concentration of L-Kynurenine was 3.28 (range 0.92-8.16) muM. The L-Kynurenine cutoff was set at 3.07 muM using receiver operating characteristics curves. The complete remission rates of patients with L-Kynurenine <3.07 and >/=3.07 muM were 69% and 51%, respectively. The 5-year overall survival (OS) rates for patients with L-Kynurenine <3.07 and >/=3.07 muM were 80.2% and 23.4%, respectively (p < 0.001). More advanced age, poor performance status, elevated lactate dehydrogenase, an unfavorable International Prognostic Index, and a poor prognostic index for T-cell lymphoma were significantly worse factors for OS. Multivariate analyses revealed only L-Kynurenine as an independent prognostic factor for OS. In conclusion, serum concentrations of L-Kynurenine might comprise a novel prognostic factor with which to determine the outcomes of treatment for PTCL-NOS. Copyright (c) 2016 John Wiley & Sons, Ltd.
Determination of l-tryptophan and l-kynurenine derivatized with (R)-4-(3-isothiocyanatopyrrolidin-1-yl)-7-(N,N-dimethylaminosulfonyl)-2,1,3-benzo xadiazole by LC-MS/MS on a triazole-bonded column and their quantification in human serum.[Pubmed:26910189]
Biomed Chromatogr. 2016 Sep;30(9):1481-6.
The concentrations of l-tryptophan (Trp) and the metabolite L-Kynurenine (KYN) can be used to evaluate the in-vivo activity of indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO). As such, a novel method involving derivatization of l-Trp and l-KYN with (R)-4-(3-isothiocyanatopyrrolidin-1-yl)-7-(N,N-dimethylaminosulfonyl)-2,1,3-benzo xadiazole (DBD-PyNCS) and separation by high-performance liquid chromatography (HPLC) with tandem mass spectrometric (MS/MS) detection on a triazole-bonded column (Cosmosil HILIC(R)) was developed to determine their concentrations. The optimized mobile phase, CH3 CN/10 mm ammonium formate in H2 O (pH 5.0) (90:10, v/v) eluted isocratically, resulted in satisfactory separation and MS/MS detection of the analytes. The detection limits of l-Trp and l-KYN were approximately 50 and 4.0 pm, respectively. The column temperature affected the retention behaviour of the Trp and KYN derivatives, with increased column temperatures leading to increased capacity factors; positive enthalpy changes were revealed by van't Hoff plot analyses. Using the proposed LC-MS/MS method, l-Trp and l-KYN were successfully determined in 10 muL human serum using 1-methyl-l-Trp as an internal standard. The precision and recovery of l-Trp were in the ranges 2.85-9.29 and 95.8-113%, respectively, while those of l-KYN were 2.51-16.0 and 80.8-98.2%, respectively. The proposed LC-MS/MS method will be useful for evaluating the in vivo activity of IDO or TDO. Copyright (c) 2016 John Wiley & Sons, Ltd.
Systemic L-Kynurenine sulfate administration disrupts object recognition memory, alters open field behavior and decreases c-Fos immunopositivity in C57Bl/6 mice.[Pubmed:26136670]
Front Behav Neurosci. 2015 Jun 16;9:157.
L-Kynurenine (L-KYN) is a central metabolite of tryptophan degradation through the kynurenine pathway (KP). The systemic administration of L-KYN sulfate (L-KYNs) leads to a rapid elevation of the neuroactive KP metabolite kynurenic acid (KYNA). An elevated level of KYNA may have multiple effects on the synaptic transmission, resulting in complex behavioral changes, such as hypoactivity or spatial working memory deficits. These results emerged from studies that focused on rats, after low-dose L-KYNs treatment. However, in several studies neuroprotection was achieved through the administration of high-dose L-KYNs. In the present study, our aim was to investigate whether the systemic administration of a high dose of L-KYNs (300 mg/bwkg; i.p.) would produce alterations in behavioral tasks (open field or object recognition) in C57Bl/6j mice. To evaluate the changes in neuronal activity after L-KYNs treatment, in a separate group of animals we estimated c-Fos expression levels in the corresponding subcortical brain areas. The L-KYNs treatment did not affect the general ambulatory activity of C57Bl/6j mice, whereas it altered their moving patterns, elevating the movement velocity and resting time. Additionally, it seemed to increase anxiety-like behavior, as peripheral zone preference of the open field arena emerged and the rearing activity was attenuated. The treatment also completely abolished the formation of object recognition memory and resulted in decreases in the number of c-Fos-immunopositive-cells in the dorsal part of the striatum and in the CA1 pyramidal cell layer of the hippocampus. We conclude that a single exposure to L-KYNs leads to behavioral disturbances, which might be related to the altered basal c-Fos protein expression in C57Bl/6j mice.
An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor.[Pubmed:21976023]
Nature. 2011 Oct 5;478(7368):197-203.
Activation of the aryl hydrocarbon receptor (AHR) by environmental xenobiotic toxic chemicals, for instance 2,3,7,8-tetrachlorodibenzo-p-dioxin (dioxin), has been implicated in a variety of cellular processes such as embryogenesis, transformation, tumorigenesis and inflammation. But the identity of an endogenous ligand activating the AHR under physiological conditions in the absence of environmental toxic chemicals is still unknown. Here we identify the tryptophan (Trp) catabolite kynurenine (Kyn) as an endogenous ligand of the human AHR that is constitutively generated by human tumour cells via tryptophan-2,3-dioxygenase (TDO), a liver- and neuron-derived Trp-degrading enzyme not yet implicated in cancer biology. TDO-derived Kyn suppresses antitumour immune responses and promotes tumour-cell survival and motility through the AHR in an autocrine/paracrine fashion. The TDO-AHR pathway is active in human brain tumours and is associated with malignant progression and poor survival. Because Kyn is produced during cancer progression and inflammation in the local microenvironment in amounts sufficient for activating the human AHR, these results provide evidence for a previously unidentified pathophysiological function of the AHR with profound implications for cancer and immune biology.


