DeoxyelephantopinCAS# 29307-03-7 |
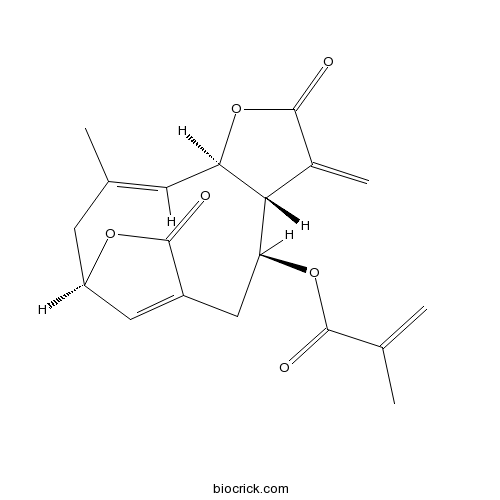
- Isodeoxyelephantopin
Catalog No.:BCN4638
CAS No.:38927-54-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29307-03-7 | SDF | Download SDF |
| PubChem ID | 6325056 | Appearance | Powder |
| Formula | C19H20O6 | M.Wt | 344.36 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1=CC2C(C(CC3=CC(C1)OC3=O)OC(=O)C(=C)C)C(=C)C(=O)O2 | ||
| Standard InChIKey | JMUOPRSXUVOHFE-GZZMZBIISA-N | ||
| Standard InChI | InChI=1S/C19H20O6/c1-9(2)17(20)24-15-8-12-7-13(23-19(12)22)5-10(3)6-14-16(15)11(4)18(21)25-14/h6-7,13-16H,1,4-5,8H2,2-3H3/b10-6+/t13-,14-,15+,16+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Deoxyelephantopin has hepatoprotective activity. 2. Deoxyelephantopin has wound healing activity. 3. Deoxyelephantopin has anti-inflammatory activity. 4. Deoxyelephantopin has antitumor activity, by inhibiting metastatic, inducing apoptosis, modulating oxidative stress , STAT3/p53/p21 signaling, MAPK pathway, PI3k/Akt/mTOR pathway, caspase cascades, and ROS . |
| Targets | ROS | Bcl-2/Bax | Caspase | p53 | p21 | PI3K | mTOR | Akt | STAT | p38MAPK | JNK | ERK | ROS | P450 (e.g. CYP17) | MMP(e.g.TIMP) | NF-kB | EGFR |

Deoxyelephantopin Dilution Calculator

Deoxyelephantopin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9039 mL | 14.5197 mL | 29.0394 mL | 58.0788 mL | 72.5984 mL |
| 5 mM | 0.5808 mL | 2.9039 mL | 5.8079 mL | 11.6158 mL | 14.5197 mL |
| 10 mM | 0.2904 mL | 1.452 mL | 2.9039 mL | 5.8079 mL | 7.2598 mL |
| 50 mM | 0.0581 mL | 0.2904 mL | 0.5808 mL | 1.1616 mL | 1.452 mL |
| 100 mM | 0.029 mL | 0.1452 mL | 0.2904 mL | 0.5808 mL | 0.726 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- L-685,458
Catalog No.:BCC2344
CAS No.:292632-98-5
- 25,26-Dihydroxyvitamin D3
Catalog No.:BCC4201
CAS No.:29261-12-9
- SB-3CT
Catalog No.:BCC5486
CAS No.:292605-14-2
- Dehydrotrametenolic acid
Catalog No.:BCN2718
CAS No.:29220-16-4
- L-Kynurenine
Catalog No.:BCC3899
CAS No.:2922-83-0
- H-DL-Phe(4-NO2)-OH
Catalog No.:BCC3279
CAS No.:2922-40-9
- Adenine HCl
Catalog No.:BCC4453
CAS No.:2922-28-3
- Methylprednisolone hemisuccinate
Catalog No.:BCC9044
CAS No.:2921-57-5
- 3',6'-Bis(diethylamino)-2-(4-nitrophenyl)spiro[isoindole-1,9'-xanthene]-3-one
Catalog No.:BCC8597
CAS No.:29199-09-5
- Shegansu B
Catalog No.:BCN3381
CAS No.:291535-65-4
- [Ala11,22,28]VIP
Catalog No.:BCC5754
CAS No.:291524-04-4
- 1-Methoxyberberine
Catalog No.:BCN7373
CAS No.:29133-52-6
- Genipin-1-O-gentiobioside
Catalog No.:BCN5349
CAS No.:29307-60-6
- Licarbazepine
Catalog No.:BCC7794
CAS No.:29331-92-8
- Tetrahydropalmatine
Catalog No.:BCN6310
CAS No.:2934-97-6
- Ciclopirox
Catalog No.:BCC4899
CAS No.:29342-05-0
- Olean-12-ene-3,11-dione
Catalog No.:BCN5195
CAS No.:2935-32-2
- H-Phg-OH
Catalog No.:BCC3310
CAS No.:2935-35-5
- SR3335
Catalog No.:BCC1964
CAS No.:293753-05-6
- T0901317
Catalog No.:BCC1178
CAS No.:293754-55-9
- Thevetiaflavone
Catalog No.:BCN4024
CAS No.:29376-68-9
- Ro 90-7501
Catalog No.:BCC7351
CAS No.:293762-45-5
- Anhydrosecoisolariciresinol
Catalog No.:BCN7521
CAS No.:29388-33-8
- Secoisolariciresinol
Catalog No.:BCN5196
CAS No.:29388-59-8
Evaluation of in vitro cytochrome P450 induction and inhibition activity of deoxyelephantopin, a sesquiterpene lactone from Elephantopus scaber L.[Pubmed:23876819]
Food Chem Toxicol. 2013 Oct;60:98-108.
Drug metabolism involving cytochrome P450 (CYP) enzymes is a key determinant of significant drug interactions. Deoxyelephantopin was evaluated for its effects on the expression of mRNAs encoding CYP1A2, CYP2D6 and CYP3A4, and protein expression and resultant enzymatic activity. The mRNA and protein expression of cytochrome isoforms were carried out using an optimized multiplex qRT-PCR assay and Western blot analysis, respectively. Human CYP3A4 protein expression was determined using an optimized hCYP3A4-HepG2 cell-based assay and the enzymatic activity was evaluated using P450-Glo CYP3A4 assay. The molecular interaction and possible inhibition of Deoxyelephantopin of the CYP3A4 enzyme was determined in silico and further validated using substrate-specific CYP3A4 inhibition assays. Deoxyelephantopin produced no significant effect on the CYP1A2 and CYP2D6 mRNA and protein expression. However, it has a weak induction effect on CYP3A4 at the transcriptional level. In silico docking simulation showed that Deoxyelephantopin has a weak interaction with CYP3A4 enzyme and it minimally affects the metabolism of CYP3A4 substrates. Deoxyelephantopin is not an in vitro CYP1A2 and CYP2D6 inducer. It is both a weak in vitro CYP3A4 inducer and inhibitor and is unlikely to elicit a clinically significant effect in human.
Anti-metastatic effect of deoxyelephantopin from Elephantopus scaber in A549 lung cancer cells in vitro.[Pubmed:25686703]
Nat Prod Res. 2015;29(24):2341-5.
In this study, we focused on the in vitro anti-metastatic effects of Deoxyelephantopin (DOE), a sesquiterpene lactone from Elephantopus scaber on lung cancer A549 cells. DOE significantly decreased the metastatic potential of A549 cells as demonstrated by transwell invasion and migration assay. DOE inhibited the expression of matrix metalloproteinase-2 (MMP-2), MMP-9, urokinase-type plasminogen activator and urokinase-type plasminogen activator receptor at transcript level. Tissue inhibitors of metalloproteinase-2 (TIMP-2) mRNA levels was up-regulated in A549 tumour cells without any change in TIMP-1 expression after DOE treatment. DOE inhibited the protein levels of p-ERK1/2 and p-Akt in A549 cells but it activated p-JNK, p-p38 protein expression. NF-kappaB and IkappaBalpha expressions were down-regulated in DOE-treated cells. All these results demonstrated that DOE has shown anti-metastatic activity against A549 tumour cells.
Antineoplastic effects of deoxyelephantopin, a sesquiterpene lactone from Elephantopus scaber, on lung adenocarcinoma (A549) cells.[Pubmed:23867245]
J Integr Med. 2013 Jul;11(4):269-77.
OBJECTIVE: Deoxyelephantopin, a sesquiterpene lactone from Elephantopus scaber, showed inhibition of the growth of various tumor cells in vitro. In the present study, we investigated the cytotoxicity and apoptosis-inducing capacity of Deoxyelephantopin on lung adenocarcinoma (A549) cells. METHODS: The cytotoxic effect of Deoxyelephantopin on A549 cells and normal lymphocytes was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and 50% inhibitory concentration (IC50) value was determined. The self-renewal and proliferating potential of A549 cells after treatment with Deoxyelephantopin were examined by colony formation assay. Cellular morphology of Deoxyelephantopin-treated cells was observed using phase-contrast microscopy. The induction of apoptosis was evaluated using acridine orange and ethidium bromide staining, Hoechst 33342 staining, terminal deoxynucleotidyl transferase-mediated dUTP biotin nick end-labeling (TUNEL) assay, DNA fragmentation analysis and Annexin V-fluorescein isothiocyanate staining by flow cytometry. Activation of caspases was detected using fluorogenic substrate specific to caspases 2, 3, 8 and 9 and flow cytometric analysis. The total cellular DNA content and expression of cleaved poly (ADP-ribose) polymerase was also analyzed. RESULTS: Deoxyelephantopin exhibited cytotoxicity to A549 cells (IC50 = 12.287 mug/mL), however, there was no toxicity towards normal human lymphocytes. Deoxyelephantopin suppressed the colony-forming ability of A549 cells in a dose-dependent manner. Acridine orange, ethidium bromide and Hoechst 33342 staining showed cell shrinkage, chromosomal condensation and nuclear fragmentation, indicating induction of apoptosis. Deoxyelephantopin increased apoptosis of A549 cells, as evidenced by more TUNEL-positive cells. DNA fragmentation and Annexin V staining revealed late-stage apoptotic cell population. Deoxyelephantopin inhibited A549 cell growth by cell cycle arrest at G2/M phase and induced apoptosis through both extrinsic and intrinsic pathways. CONCLUSION: These results suggest that Deoxyelephantopin has great potential as a new chemotherapeutic agent to be developed further for the treatment of lung cancer.
Deoxyelephantopin impairs growth of cervical carcinoma SiHa cells and induces apoptosis by targeting multiple molecular signaling pathways.[Pubmed:25260383]
Cell Biol Toxicol. 2014 Dec;30(6):331-43.
Deoxyelephantopin, a sesquiterpene lactone extracted and purified from Elephantopus scaber, has been shown to exhibit antitumor and hepatoprotective activities. The purpose of this study was to investigate the antiproliferative and apoptosis-inducing properties of Deoxyelephantopin in SiHa cells and to elucidate the underlying molecular mechanisms. Deoxyelephantopin inhibited growth of SiHa cells and triggered apoptosis. Apoptosis was accompanied by sequential activation of caspases (8, 9, 3, and 7) and reactive oxygen species (ROS) production. Downregulation of antiapoptotic proteins (Bcl2 and Bcl-xL) and upregulation of apoptotic protein (bax) were also detected. Our results demonstrated that Deoxyelephantopin-induced G2/M phase arrest was associated with a marked increase in the levels of p53 and p21 and a decrease in phospho-signal transducer and activator of transcription 3 (pSTAT3-Tyr705), cyclin-dependent kinase 1 (cdc2), and cyclin B1. The expression of p-Akt and p-mTOR was downregulated. p-ERK was inhibited while p-JNK and p-p38 was activated on Deoxyelephantopin treatment. Our findings provided the first evidence that STAT3/p53/p21 signaling, MAPK pathway, PI3k/Akt/mTOR pathway, caspase cascades, and ROS play critical roles in Deoxyelephantopin-induced G2/M phase arrest and apoptosis of SiHa cells.
Deoxyelephantopin impedes mammary adenocarcinoma cell motility by inhibiting calpain-mediated adhesion dynamics and inducing reactive oxygen species and aggresome formation.[Pubmed:22342517]
Free Radic Biol Med. 2012 Apr 15;52(8):1423-36.
We previously showed that Deoxyelephantopin (DET), a plant sesquiterpene lactone, exhibits more profound suppression than paclitaxel (PTX) of lung metastasis of mammary adenocarcinoma TS/A cells in mice. Proteomics studies suggest that DET affects actin cytoskeletal protein networks and downregulates calpain-mediated proteolysis of several actin-associated proteins, whereas PTX mainly interferes with microtubule proteins. Here, DET was observed to significantly deregulate adhesion formation in TS/A cells, probably through inhibition of m-calpain activity. Epithelial growth factor (EGF)-mediated activation of Rho GTPase Rac1 and formation of lamellipodia in TS/A cells were remarkably suppressed by DET treatment. Further, DET impaired vesicular trafficking of EGF and induced protein carbonylation and formation of centrosomal aggregates in TS/A cells. DET-induced reactive oxygen species were observed to be the upstream stimulus for the formation of centrosomal ubiquitinated protein aggregates that might subsequently restrict cancer cell motility. PTX, however, caused dramatic morphological changes, interfered with microtubule networking, and moderately inhibited calpain-mediated cytoskeletal and focal adhesion protein cleavage in TS/A cells. This study provides novel mechanistic insights into the pharmacological action of DET against metastatic mammary cell migration and suggests that modulation of oxidative stress might be a potential strategy for treatment of metastatic breast cancer.


