SecoisolariciresinolCAS# 29388-59-8 |
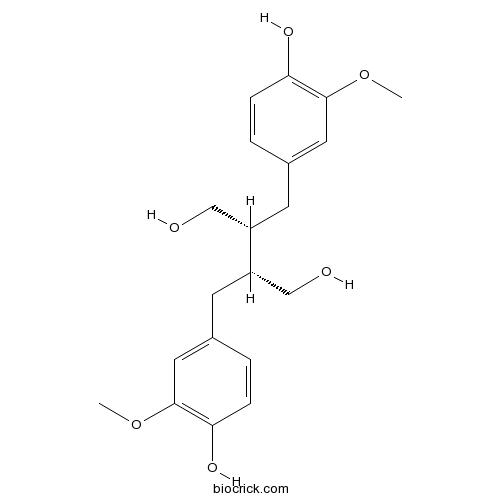
- (+)-Secoisolariciresinol
Catalog No.:BCN9042
CAS No.:145265-02-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29388-59-8 | SDF | Download SDF |
| PubChem ID | 65373 | Appearance | Powder |
| Formula | C20H26O6 | M.Wt | 362.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3R)-2,3-bis[(4-hydroxy-3-methoxyphenyl)methyl]butane-1,4-diol | ||
| SMILES | COC1=C(C=CC(=C1)CC(CO)C(CC2=CC(=C(C=C2)O)OC)CO)O | ||
| Standard InChIKey | PUETUDUXMCLALY-HOTGVXAUSA-N | ||
| Standard InChI | InChI=1S/C20H26O6/c1-25-19-9-13(3-5-17(19)23)7-15(11-21)16(12-22)8-14-4-6-18(24)20(10-14)26-2/h3-6,9-10,15-16,21-24H,7-8,11-12H2,1-2H3/t15-,16-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Secoisolariciresinol and matairesinol are enterolignan precursors. 2. Secoisolariciresinol has antioxidant activity. 3. Secoisolariciresinol and isotaxiresinol prevent d-GalN/LPS-induced hepatic injury by inhibiting hepatocyte apoptosis through the blocking of TNF-alpha and IFN-gamma production by activated macrophages and direct inhibition of the apoptosis induced by TNF-alpha. 4. Secoisolariciresinol has estrogen-like activity, it can significantly suppress triglyceride (TG) accumulation in 3T3-L1 adipocytes. 5. (-)-Secoisolariciresinol exerts a suppressive effect on the gain of body weight of mice fed a high-fat diet by inducing gene expression of adiponectin, resulting in the altered expression of various genes related to the synthesis and β-oxidation of fatty acids. |
| Targets | IFN-γ | TNF-α | Estrogen receptor | Progestogen receptor |

Secoisolariciresinol Dilution Calculator

Secoisolariciresinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7594 mL | 13.7969 mL | 27.5938 mL | 55.1876 mL | 68.9845 mL |
| 5 mM | 0.5519 mL | 2.7594 mL | 5.5188 mL | 11.0375 mL | 13.7969 mL |
| 10 mM | 0.2759 mL | 1.3797 mL | 2.7594 mL | 5.5188 mL | 6.8985 mL |
| 50 mM | 0.0552 mL | 0.2759 mL | 0.5519 mL | 1.1038 mL | 1.3797 mL |
| 100 mM | 0.0276 mL | 0.138 mL | 0.2759 mL | 0.5519 mL | 0.6898 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Anhydrosecoisolariciresinol
Catalog No.:BCN7521
CAS No.:29388-33-8
- Ro 90-7501
Catalog No.:BCC7351
CAS No.:293762-45-5
- Thevetiaflavone
Catalog No.:BCN4024
CAS No.:29376-68-9
- T0901317
Catalog No.:BCC1178
CAS No.:293754-55-9
- SR3335
Catalog No.:BCC1964
CAS No.:293753-05-6
- H-Phg-OH
Catalog No.:BCC3310
CAS No.:2935-35-5
- Olean-12-ene-3,11-dione
Catalog No.:BCN5195
CAS No.:2935-32-2
- Ciclopirox
Catalog No.:BCC4899
CAS No.:29342-05-0
- Tetrahydropalmatine
Catalog No.:BCN6310
CAS No.:2934-97-6
- Licarbazepine
Catalog No.:BCC7794
CAS No.:29331-92-8
- Genipin-1-O-gentiobioside
Catalog No.:BCN5349
CAS No.:29307-60-6
- Deoxyelephantopin
Catalog No.:BCN4655
CAS No.:29307-03-7
- Cyclen
Catalog No.:BCN8441
CAS No.:294-90-6
- 7-Nitroindazole
Catalog No.:BCC6713
CAS No.:2942-42-9
- 4',7-Di-O-methylnaringenin
Catalog No.:BCN5197
CAS No.:29424-96-2
- Pseudolycorine
Catalog No.:BCN5371
CAS No.:29429-03-6
- Sophorabioside
Catalog No.:BCN7838
CAS No.:2945-88-2
- Ticarcillin sodium
Catalog No.:BCC4737
CAS No.:29457-07-6
- L 006235
Catalog No.:BCC2361
CAS No.:294623-49-7
- Ganoderic acid Z
Catalog No.:BCN2440
CAS No.:294674-09-2
- Narciclasine
Catalog No.:BCN4732
CAS No.:29477-83-6
- Cypellocarpin C
Catalog No.:BCN7556
CAS No.:294856-66-9
- Valerosidate
Catalog No.:BCN6750
CAS No.:29505-31-5
- MNI-caged-L-glutamate
Catalog No.:BCC7086
CAS No.:295325-62-1
Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans.[Pubmed:24429845]
Food Funct. 2014 Mar;5(3):491-501.
Secoisolariciresinol-diglycoside (SDG), a natural dietary lignan of flaxseeds now available in dietary supplements, is converted by intestinal bacteria to the mammalian lignans enterodiol and enterolactone. High levels of these lignans in blood and urine are associated with reduced risk of many chronic diseases. Our objective was to determine the bioavailability and pharmacokinetics of SDG in purified flaxseed extracts under dose-ranging and steady-state conditions, and to examine whether differences in Secoisolariciresinol-diglycoside purity influence bioavailability. Pharmacokinetic studies were performed on healthy postmenopausal women after oral intake of 25, 50, 75, 86 and 172 mg of Secoisolariciresinol-diglycoside. Extracts differing in Secoisolariciresinol-diglycoside purity were compared, and steady-state lignan concentrations measured after daily intake for one week. Blood and urine samples were collected at timed intervals and Secoisolariciresinol, enterodiol and enterolactone concentrations measured by mass spectrometry. Secoisolariciresinol-diglycoside was efficiently hydrolyzed and converted to Secoisolariciresinol. Serum concentrations increased rapidly after oral intake, peaking after 5-7 h and disappearing with a plasma elimination half-life of 4.8 h. Maximum serum concentrations of the biologically active metabolites, enterodiol and enterolactone were attained after 12-24 h and 24-36 h, respectively, and the half-lives were 9.4 h and 13.2 h. Linear dose-responses were observed and Secoisolariciresinol bioavailability correlated (r(2) = 0.835) with cumulative lignan excretion. There were no significant differences in the pharmacokinetics of extracts differing in purity, and steady-state serum lignan concentrations were obtained after one-week of daily dosing. In conclusion, this study defines the pharmacokinetics of Secoisolariciresinol-diglycoside and shows it is first hydrolyzed and then metabolized in a time-dependent sequence to Secoisolariciresinol, enterodiol and ultimately enterolactone, and these metabolites are efficiently absorbed.
Secoisolariciresinol and isotaxiresinol inhibit tumor necrosis factor-alpha-dependent hepatic apoptosis in mice.[Pubmed:15043992]
Life Sci. 2004 Apr 16;74(22):2781-92.
The effects of Secoisolariciresinol (1) and isotaxiresinol (2), two major lignans isolated from the wood of Taxus yunnanensis, on tumor necrosis factor-alpha (TNF-alpha)-dependent hepatic apoptosis induced by D-galactosamine (d-GalN)/lipopolysaccharide (LPS) were investigated in mice. Co-administration of d-GalN (700 mg/kg) and LPS (10 microg/kg) resulted in a typical hepatic apoptosis characterized by DNA fragmentation and the formation of apoptotic bodies. Serum glutamic pyruvic transaminase (sGPT) and glutamic oxaloacetic transaminase (sGOT) levels were also raised at 8 h after d-GalN/LPS intoxication due to a severe necrosis of hepatocytes. Pre-administration of 1 or 2 (50, 10 mg/kg, i.p.) 12 and 1 h before d-GalN/LPS significantly reduced DNA fragmentation and prevented chromatin condensation, apoptotic body formation and hepatitis. Pro-inflammatory cytokines such as TNF-alpha and interferon-gamma (IFN-gamma) secreted from LPS-activated macrophages are important mediators of hepatocyte apoptosis in this model. Pre-treatment with 1 or 2 significantly inhibited the elevation of serum TNF-alpha and IFN-gamma levels. In a separate experiment, both lignans had a significant dose-dependent protective effect on d-GalN/TNF-alpha-induced cell death in primary cultured mouse hepatocytes and TNF-alpha-mediated cell death in murine L929 fibrosarcoma cells. These results indicated that 1 and 2 prevent d-GalN/LPS-induced hepatic injury by inhibiting hepatocyte apoptosis through the blocking of TNF-alpha and IFN-gamma production by activated macrophages and direct inhibition of the apoptosis induced by TNF-alpha.
Antioxidant Activity of Secoisolariciresinol Diglucoside-derived Metabolites, Secoisolariciresinol, Enterodiol, and Enterolactone.[Pubmed:11062311]
Int J Angiol. 2000 Oct;9(4):220-225.
Secoisolariciresinol diglucoside (SDG), an antioxidant isolated from flaxseed, is metabolized to Secoisolariciresinol (SECO), enterodiol (ED), and enterolactone (EL) in the body. The effectiveness of SDG in hypercholesterolemic atherosclerosis, diabetes, and endotoxic shock could be due to these metabolites. These metabolites may have antioxidant activity. However, the antioxidant activity of these metabolites is not known. The antioxidant activity of SECO, ED, and EL was investigated using chemiluminescence (CL) of zymosan-activated polymorphonuclear leukocytes (PMNLs) [PMNL-CL]. Other antioxidants (SDG and vitamin E) were also used for comparison. SDG, SECO, ED, EL, and vitamin E, each in the concentration of 0.5, 1.0, 2.5, 5.0 and 10.0 mg/ml, produced a concentration-dependent reduction in zymosan-activated PMNL-CL. SDG, SECO, ED, EL, and vitamin E, in the concentration of 2.5 mg/ml, produced a reduction of zymosan-activated PMNL-CL by 23.8%, 91.2%, 94.2%, 81.6% and 18.7%, respectively. Activated PMNLs produce reactive oxygen species and luminol-dependent CL reflects the amount of oxygen species generated from activated PMNLs. The reduction of PMNL-CL, therefore, reflects the antioxidant activity of the compounds studied. These results suggest that the metabolites of SDG have antioxidant activity. The antioxidant activity was highest with SECO and ED and lowest with vitamin E. The antioxidant potency of SECO, ED, EL, and SDG was 4.86, 5.02, 4.35, and 1.27 respectively, as compared to vitamin E. SECO, ED and EL are respectively 3.82, 3.95, and 3.43 more potent than SDG.
Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol.[Pubmed:15877880]
Br J Nutr. 2005 Mar;93(3):393-402.
Enterolignans (enterodiol and enterolactone) can potentially reduce the risk of certain cancers and cardiovascular diseases. Enterolignans are formed by the intestinal microflora after the consumption of plant lignans. Until recently, only Secoisolariciresinol and matairesinol were considered enterolignan precursors, but now several new precursors have been identified, of which lariciresinol and pinoresinol have a high degree of conversion. Quantitative data on the contents in foods of these new enterolignan precursors are not available. Thus, the aim of this study was to compile a lignan database including all four major enterolignan precursors. Liquid chromatography-tandem mass spectrometry was used to quantify lariciresinol, pinoresinol, Secoisolariciresinol and matairesinol in eighty-three solid foods and twenty-six beverages commonly consumed in The Netherlands. The richest source of lignans was flaxseed (301,129 microg/100 g), which contained mainly Secoisolariciresinol. Also, lignan concentrations in sesame seeds (29,331 microg/100 g, mainly pinoresinol and lariciresinol) were relatively high. For grain products, which are known to be important sources of lignan, lignan concentrations ranged from 7 to 764 microg/100 g. However, many vegetables and fruits had similar concentrations, because of the contribution of lariciresinol and pinoresinol. Brassica vegetables contained unexpectedly high levels of lignans (185-2321 microg/100 g), mainly pinoresinol and lariciresinol. Lignan levels in beverages varied from 0 (cola) to 91 microg/100 ml (red wine). Only four of the 109 foods did not contain a measurable amount of lignans, and in most cases the amount of lariciresinol and pinoresinol was larger than that of Secoisolariciresinol and matairesinol. Thus, available databases largely underestimate the amount of enterolignan precursors in foods.
(-)-Secoisolariciresinol attenuates high-fat diet-induced obesity in C57BL/6 mice.[Pubmed:22030618]
Food Funct. 2012 Jan;3(1):76-82.
Flaxseed lignan, Secoisolariciresinol has been reported to possess health benefits. We previously synthesized each stereoisomer of Secoisolariciresinol and found that (-)-Secoisolariciresinol reduces lipid accumulation and induces adiponectin production in 3T3-L1 adipocytes. Here we show the effects of (-)-Secoisolariciresinol on high-fat diet-induced obesity in C57BL/6 male mice. Oral administration of (-)-Secoisolariciresinol for 28 consecutive days significantly suppressed the gain of body weight. Increased serum adiponectin level and decreased gene expression of fatty acid synthase and sterol regulatory element-binding protein-1c in liver, which are related to fatty acid synthesis, were observed in the mice orally administered with (-)-Secoisolariciresinol. In addition, subcutaneous injection of (-)-Secoisolariciresinol also significantly suppressed the gain of body weight. Serum leptin levels were significantly increased by treating with (-)-Secoisolariciresinol or (-)-enterolactone. Subcutaneous injection of (-)-Secoisolariciresinol, (-)-enterolactone, or (-)-enterodiol promoted gene expression of acyl-CoA oxidase, carnitine palmitoyl transferase-1, and peroxisome proliferator-activated receptor alpha, which are related to beta-oxidation. Overall results suggest that (-)-Secoisolariciresinol exerts a suppressive effect on the gain of body weight of mice fed a high-fat diet by inducing gene expression of adiponectin, resulting in the altered expression of various genes related to the synthesis and beta-oxidation of fatty acids.
The Effect of Secoisolariciresinol on 3T3-L1 Adipocytes and the Relationship between Molecular Structure and Activity.[Pubmed:19129664]
Biosci Biotechnol Biochem. 2009 Jan;73(1):35-9.
As we have reported, flaxseed lignan, (+)-Secoisolariciresinol (SECO), (-)-SECO, and meso-SECO were stereoselectively synthesized and their biological functions were evaluated. In the present study, we focused on the effects of SECOs on the regulation of 3T3-L1 adipocytes, and identified the structure-activity relationships. Optically active SECO and meso-SECO were tested for their effects on lipid metabolism in 3T3-L1 adipocytes. (-)-SECO accelerated adiponectin production of 3T3-L1 adipocytes. On the other hand, (+)- and meso-SECO suppressed the production of adiponectin. In addition, triglyceride (TG) accumulation in 3T3-L1 adipocytes was significantly suppressed by all three SECOs tested here, as was 17beta-estradiol, when the SECOs were added to the medium during induction of 3T3-L1 preadipocytes to adipocytes. Especially, (-)-SECO strongly reduced TG accumulation. It is well-known that SECO has estrogen-like activity. Hence the estrogen-like activity of each SECO compound was assessed. Only (-)-SECO had estrogen-like activity.


