PseudolycorineCAS# 29429-03-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29429-03-6 | SDF | Download SDF |
| PubChem ID | 443689 | Appearance | Powder |
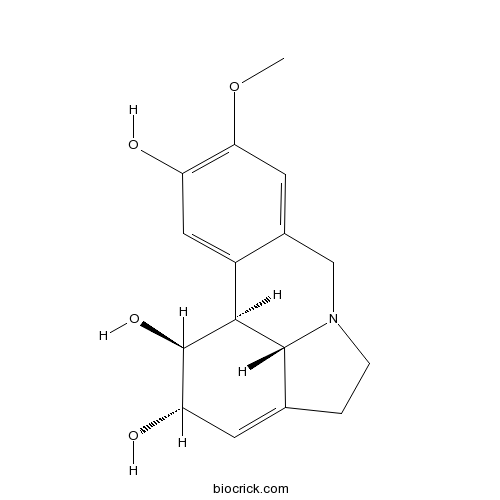
| Formula | C16H19NO4 | M.Wt | 289.33 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | COC1=C(C=C2C3C(C(C=C4C3N(CC4)CC2=C1)O)O)O | ||
| Standard InChIKey | CKAHWDNDUGDSLE-ARLBYUKCSA-N | ||
| Standard InChI | InChI=1S/C16H19NO4/c1-21-13-5-9-7-17-3-2-8-4-12(19)16(20)14(15(8)17)10(9)6-11(13)18/h4-6,12,14-16,18-20H,2-3,7H2,1H3/t12-,14-,15+,16+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Pseudolycorine can halt HeLa cell growth at 10-1 mM or lower concentrations, it at its growth inhibitory concentrations blocks protein synthesis in ascites cells and stabilize HeLa cell polysomes in vivo. 2. Pseudolycorine exhibits cytotoxic profiles against cancer cell lines. 3. Pseudolycorine and haemanthamine show good activity in in vitro assays against Trypanosoma brucei rhodesiense, T. cruzi and Plasmodium falciparum with IC50 values in the range of 3.66 uM or lower. 4. Pseudolycorine, primarily studied as a new antiviral agent , it also shows remarkable antileukemic activity. |
| Targets | Antifection | DNA/RNA Synthesis |

Pseudolycorine Dilution Calculator

Pseudolycorine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4563 mL | 17.2813 mL | 34.5626 mL | 69.1252 mL | 86.4065 mL |
| 5 mM | 0.6913 mL | 3.4563 mL | 6.9125 mL | 13.825 mL | 17.2813 mL |
| 10 mM | 0.3456 mL | 1.7281 mL | 3.4563 mL | 6.9125 mL | 8.6407 mL |
| 50 mM | 0.0691 mL | 0.3456 mL | 0.6913 mL | 1.3825 mL | 1.7281 mL |
| 100 mM | 0.0346 mL | 0.1728 mL | 0.3456 mL | 0.6913 mL | 0.8641 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4',7-Di-O-methylnaringenin
Catalog No.:BCN5197
CAS No.:29424-96-2
- 7-Nitroindazole
Catalog No.:BCC6713
CAS No.:2942-42-9
- Cyclen
Catalog No.:BCN8441
CAS No.:294-90-6
- Secoisolariciresinol
Catalog No.:BCN5196
CAS No.:29388-59-8
- Anhydrosecoisolariciresinol
Catalog No.:BCN7521
CAS No.:29388-33-8
- Ro 90-7501
Catalog No.:BCC7351
CAS No.:293762-45-5
- Thevetiaflavone
Catalog No.:BCN4024
CAS No.:29376-68-9
- T0901317
Catalog No.:BCC1178
CAS No.:293754-55-9
- SR3335
Catalog No.:BCC1964
CAS No.:293753-05-6
- H-Phg-OH
Catalog No.:BCC3310
CAS No.:2935-35-5
- Olean-12-ene-3,11-dione
Catalog No.:BCN5195
CAS No.:2935-32-2
- Ciclopirox
Catalog No.:BCC4899
CAS No.:29342-05-0
- Sophorabioside
Catalog No.:BCN7838
CAS No.:2945-88-2
- Ticarcillin sodium
Catalog No.:BCC4737
CAS No.:29457-07-6
- L 006235
Catalog No.:BCC2361
CAS No.:294623-49-7
- Ganoderic acid Z
Catalog No.:BCN2440
CAS No.:294674-09-2
- Narciclasine
Catalog No.:BCN4732
CAS No.:29477-83-6
- Cypellocarpin C
Catalog No.:BCN7556
CAS No.:294856-66-9
- Valerosidate
Catalog No.:BCN6750
CAS No.:29505-31-5
- MNI-caged-L-glutamate
Catalog No.:BCC7086
CAS No.:295325-62-1
- Eupatoletin
Catalog No.:BCN3605
CAS No.:29536-44-5
- Olivil
Catalog No.:BCN5198
CAS No.:2955-23-9
- Negletein
Catalog No.:BCN8085
CAS No.:29550-13-8
- (E)-N-Caffeoylputrescine
Catalog No.:BCC8391
CAS No.:29554-26-5
Inhibitors of protein synthesis in eukarytic cells. Comparative effects of some amaryllidaceae alkaloids.[Pubmed:944052]
Biochim Biophys Acta. 1976 Mar 17;425(3):342-8.
The effects of eighteen compounds obtained from bulbs of the Amaryllidaceae family were tested on (a) animal cell growth, (b) DNA, RNA and protein synthesis by intact cells and (c) protein synthesis in cell-free systems. Dihydrolycorine, haemanthamine, lycorine, narciclasine, pretazettine and Pseudolycorine halted HeLa cell growth at 10(-1) mM or lower concentrations. These compounds at their growth inhibitory concentrations block protein synthesis in ascites cells and stabilize HeLa cell polysomes in vivo. Endomyocarditis virus RNA-directed cell-free polypeptide synthesis by an ascites S-30 extract and acetyl-[14C]leucyl-puromycin formation by ascites ribosomes are also inhibited by the six compounds indicated above. It is therefore concluded that they halt protein synthesis in eukaryotic cells by inhibiting the peptide bone formation step.
Amaryllidaceae alkaloids belonging to different structural subgroups display activity against apoptosis-resistant cancer cells.[Pubmed:20550100]
J Nat Prod. 2010 Jul 23;73(7):1223-7.
Fifteen Amaryllidaceae alkaloids (1-15) were evaluated for their antiproliferative activities against six distinct cancer cell lines. Several of these natural products were found to have low micromolar antiproliferative potencies. The log P values of these compounds did not influence their observed activity. When active, the compounds displayed cytostatic, not cytotoxic activity, with the exception of Pseudolycorine (3), which exhibited cytotoxic profiles. The active compounds showed similar efficacies toward cancer cells irrespective of whether the cell lines were responsive or resistant to proapoptotic stimuli. Altogether, the data from the present study revealed that lycorine (1), amarbellisine (6), haemanthamine (14), and haemanthidine (15) are potentially useful chemical scaffolds to generate further compounds to combat cancers associated with poor prognoses, especially those naturally resistant to apoptosis, such as glioblastoma, melanoma, non-small-cell lung, and metastatic cancers.


