MNI-caged-L-glutamateStable photoreleaser of L-glutamate CAS# 295325-62-1 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- Nocodazole
Catalog No.:BCC3826
CAS No.:31430-18-9
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- Mc-MMAE
Catalog No.:BCC5201
CAS No.:863971-24-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 295325-62-1 | SDF | Download SDF |
| PubChem ID | 6604871 | Appearance | Powder |
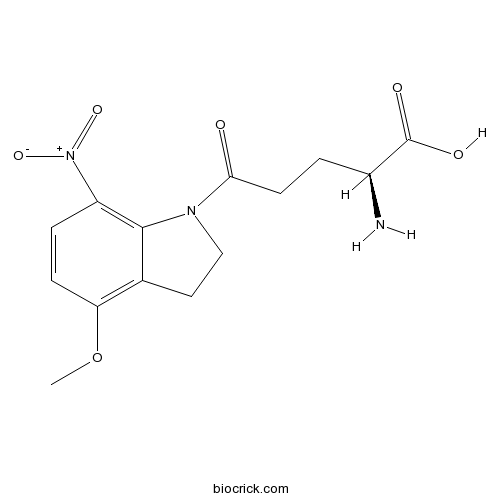
| Formula | C14H17N3O6 | M.Wt | 323.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 4-Methoxy-7-nitroindolinyl-caged-L-glutamate, MNI glutamate | ||
| Solubility | Soluble to 50 mM in water with gentle warming | ||
| Chemical Name | (2S)-2-amino-5-(4-methoxy-7-nitro-2,3-dihydroindol-1-yl)-5-oxopentanoic acid | ||
| SMILES | COC1=C2CCN(C2=C(C=C1)[N+](=O)[O-])C(=O)CCC(C(=O)O)N | ||
| Standard InChIKey | GXIDBZKXGUNITQ-VIFPVBQESA-N | ||
| Standard InChI | InChI=1S/C14H17N3O6/c1-23-11-4-3-10(17(21)22)13-8(11)6-7-16(13)12(18)5-2-9(15)14(19)20/h3-4,9H,2,5-7,15H2,1H3,(H,19,20)/t9-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | MNI-caged glutamate that rapidly and efficiently releases glutamate when photolysed (300 - 380 nm excitation). Water-soluble, highly resistant to hydrolysis, stable at neutral pH, and pharmacologically inactive at neuronal glutamate receptors (up to mM concentrations). 2.5-fold more efficient at releasing L-glutamate than NI-caged L-glutamate. |

MNI-caged-L-glutamate Dilution Calculator

MNI-caged-L-glutamate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0931 mL | 15.4655 mL | 30.931 mL | 61.862 mL | 77.3276 mL |
| 5 mM | 0.6186 mL | 3.0931 mL | 6.1862 mL | 12.3724 mL | 15.4655 mL |
| 10 mM | 0.3093 mL | 1.5466 mL | 3.0931 mL | 6.1862 mL | 7.7328 mL |
| 50 mM | 0.0619 mL | 0.3093 mL | 0.6186 mL | 1.2372 mL | 1.5466 mL |
| 100 mM | 0.0309 mL | 0.1547 mL | 0.3093 mL | 0.6186 mL | 0.7733 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Valerosidate
Catalog No.:BCN6750
CAS No.:29505-31-5
- Cypellocarpin C
Catalog No.:BCN7556
CAS No.:294856-66-9
- Narciclasine
Catalog No.:BCN4732
CAS No.:29477-83-6
- Ganoderic acid Z
Catalog No.:BCN2440
CAS No.:294674-09-2
- L 006235
Catalog No.:BCC2361
CAS No.:294623-49-7
- Ticarcillin sodium
Catalog No.:BCC4737
CAS No.:29457-07-6
- Sophorabioside
Catalog No.:BCN7838
CAS No.:2945-88-2
- Pseudolycorine
Catalog No.:BCN5371
CAS No.:29429-03-6
- 4',7-Di-O-methylnaringenin
Catalog No.:BCN5197
CAS No.:29424-96-2
- 7-Nitroindazole
Catalog No.:BCC6713
CAS No.:2942-42-9
- Cyclen
Catalog No.:BCN8441
CAS No.:294-90-6
- Secoisolariciresinol
Catalog No.:BCN5196
CAS No.:29388-59-8
- Eupatoletin
Catalog No.:BCN3605
CAS No.:29536-44-5
- Olivil
Catalog No.:BCN5198
CAS No.:2955-23-9
- Negletein
Catalog No.:BCN8085
CAS No.:29550-13-8
- (E)-N-Caffeoylputrescine
Catalog No.:BCC8391
CAS No.:29554-26-5
- Sakuranetin
Catalog No.:BCN5199
CAS No.:2957-21-3
- 2-Amino-2',5-dichlorobenzophenone
Catalog No.:BCC8520
CAS No.:2958-36-3
- Gynuramide II
Catalog No.:BCN5200
CAS No.:295803-03-1
- Friedelin 3,4-lactone
Catalog No.:BCN6449
CAS No.:29621-75-8
- 13-Oxo-9E,11E-octadecadienoic acid
Catalog No.:BCN8173
CAS No.:29623-29-8
- 2-Aminothiazol-4-acetic acid
Catalog No.:BCC8556
CAS No.:29676-71-9
- Ethynodiol diacetate
Catalog No.:BCC4483
CAS No.:297-76-7
- Oxyresveratrol
Catalog No.:BCN5201
CAS No.:29700-22-9
New caged neurotransmitter analogs selective for glutamate receptor sub-types based on methoxynitroindoline and nitrophenylethoxycarbonyl caging groups.[Pubmed:22609535]
Neuropharmacology. 2012 Sep;63(4):624-34.
Photolysis is widely used in experimental neuroscience to isolate post-synaptic receptor activation from presynaptic processes, to determine receptor mechanisms in situ, for pharmacological dissection of signaling pathways, or for photostimulation/inhibition in neural networks. We have evaluated new caged neuroactive amino acids that use 4-methoxy-7-nitroindolinyl- (MNI) or 1-(2-nitrophenyl)ethoxycarbonyl (NPEC) photoprotecting groups to make caged ligands specific for glutamate receptor sub-types. Each was tested for interference with synaptic transmission and excitability and for receptor-specific actions in slice preparations. No adverse effects were found at glutamate receptors. At high concentration, MNI-caged, but not NPEC-caged ligands, interfered with GABA-ergic transmission. MNI-caged amino acids have sub-microsecond release times suitable for investigating mechanisms at fast synaptic receptors in situ. MNI-NMDA and MNI-kainate were synthesized and tested. MNI-NMDA showed stoichiometric release of chirally pure NMDA. Wide-field photolysis in cerebellar interneurons produced a fast-rising sustained activation of NMDA receptors, and localized laser photolysis gave a fast, transient response. Photolysis of MNI-kainate to release up to 4 muM kainate generated large inward currents at resting membrane potential in Purkinje neurons. Application of GYKI 53655 indicated that 40% of the current was due to AMPA receptor activation by kainate. Signaling via metabotropic glutamate receptors (mGluR) does not require fast release rates. NPEC cages are simpler to prepare but have slower photorelease. Photolysis of NPEC-ACPD or NPEC-DHPG in Purkinje neurons generated slow inward currents blocked by the mGluR type 1 antagonist CPCCOEt similar to the slow sEPSC seen with parallel fiber burst stimulation. NPEC-AMPA was also tested in Purkinje neurons and showed large sustained inward currents selective for AMPA receptors with little activation of kainate receptors. MNI-caged l-glutamate, NMDA and kainate inhibit GABA-A receptors with IC(5)(0) concentrations close to the maximum concentrations useful in receptor signaling experiments.
Comparative analysis of inhibitory effects of caged ligands for the NMDA receptor.[Pubmed:15652611]
J Neurosci Methods. 2005 Mar 15;142(1):1-9.
Photolytic release of neurotransmitters from caged precursors is a useful method to study synaptic processes with high temporal and spatial resolution. At present, the two most widely used classes of caged precursors for studies on glutamate receptors are based on derivatives of the 2-nitrobenzyl caging group (alpha-carboxy-2-nitrobenzyl, CNB) and the nitroindoline caging group (7-nitroindoline, NI, and 4-methoxy-7-nitroindoline, MNI). Besides NI- and MNI-caged amino acids being thermally more stable than the CNB-caged amino acids, there have been no other major advantages reported of using compounds from either of these two classes. Here, we show inhibitory effects of CNB-glutamate and a number of other CNB-caged agonists on N-methyl-D-aspartate (NMDA) receptors at non-saturating concentrations of the co-agonist glycine. In contrast, NI- and MNI-glutamate and most other NI-/MNI-caged agonists that we tested were inert under these conditions. Furthermore, we demonstrate that carboxynitroindoline-caged glycine (CNI-glycine), which was previously found to inhibit glycine receptors, has no such effect on NMDA receptors. Together, these findings underline the usefulness of NI- and MNI-caged ligands and show that CNB-caged compounds should be avoided in studies involving NMDA receptors.
Photochemical and pharmacological evaluation of 7-nitroindolinyl-and 4-methoxy-7-nitroindolinyl-amino acids as novel, fast caged neurotransmitters.[Pubmed:11640955]
J Neurosci Methods. 2001 Nov 15;112(1):29-42.
Reagents capable of rapid and efficient release of neuroactive amino acids (L-glutamate, GABA and glycine) upon flash photolysis of thermally stable, inert precursors have been elusive. 7-Nitroindolinyl (NI)-caged and 4-methoxy-7-nitroindolinyl (MNI)-caged compounds that fulfil these criteria are evaluated here. These caged precursors are highly resistant to hydrolysis. Photolysis is fast (half time< or =0.26 ms) and the conversion achieved with a xenon flashlamp is about 15% for the NI-caged L-glutamate and about 35% for the MNI-caged L-glutamate. A procedure is described for calibration of photolysis in a microscope-based experimental apparatus. NI-caged L-glutamate itself showed no agonist or antagonist effects on AMPA and NMDA receptors in cultured neurones, and had no effect on climbing fibre activation of Purkinje neurones. A control compound with identical photochemistry that generated an inert phosphate upon photolysis was used to confirm that the intermediates and by-products of photolysis have no deleterious effects. MNI-caged L-glutamate is as stable and fast as NI-caged L-glutamate and similarly inert at glutamate receptors, but about 2.5 times more efficient. However, NI-caged GABA is an antagonist at GABA(A) receptors and NI-glycine an antagonist at glycine receptors. The results show the utility and limitations of these fast and stable caged neurotransmitters in the investigation of synaptic processes.
Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons.[Pubmed:11687814]
Nat Neurosci. 2001 Nov;4(11):1086-92.
Dendritic spines serve as preferential sites of excitatory synaptic connections and are pleomorphic. To address the structure-function relationship of the dendritic spines, we used two-photon uncaging of glutamate to allow mapping of functional glutamate receptors at the level of the single synapse. Our analyses of the spines of CA1 pyramidal neurons reveal that AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)-type glutamate receptors are abundant (up to 150/spine) in mushroom spines but sparsely distributed in thin spines and filopodia. The latter may be serving as the structural substrates of the silent synapses that have been proposed to play roles in development and plasticity of synaptic transmission. Our data indicate that distribution of functional AMPA receptors is tightly correlated with spine geometry and that receptor activity is independently regulated at the level of single spines.


