NocodazoleTubulin production inhibitor,anti-neoplastic agent CAS# 31430-18-9 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- MMAD
Catalog No.:BCC1774
CAS No.:203849-91-6
- Eribulin
Catalog No.:BCC5174
CAS No.:253128-41-5
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- Mc-MMAE
Catalog No.:BCC5201
CAS No.:863971-24-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

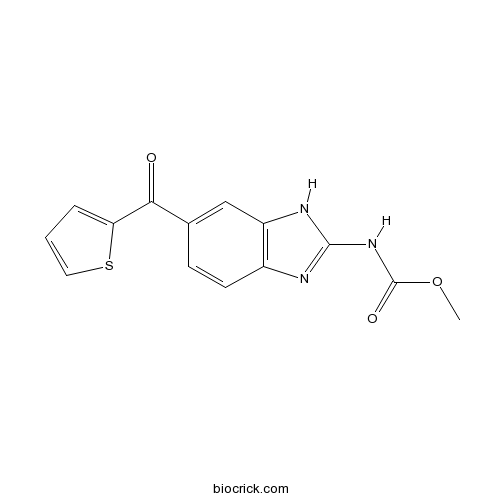
| Cas No. | 31430-18-9 | SDF | Download SDF |
| PubChem ID | 4122 | Appearance | Powder |
| Formula | C14H11N3O3S | M.Wt | 301.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Oncodazole; R17934 | ||
| Solubility | DMSO : 20 mg/mL (66.37 mM; Need ultrasonic) | ||
| Chemical Name | methyl N-[6-(thiophene-2-carbonyl)-1H-benzimidazol-2-yl]carbamate | ||
| SMILES | COC(=O)NC1=NC2=C(N1)C=C(C=C2)C(=O)C3=CC=CS3 | ||
| Standard InChIKey | KYRVNWMVYQXFEU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H11N3O3S/c1-20-14(19)17-13-15-9-5-4-8(7-10(9)16-13)12(18)11-3-2-6-21-11/h2-7H,1H3,(H2,15,16,17,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Microtubule inhibitor; inhibits mitosis. Also inhibits autophagosome-lysosome fusion. Enhances homology-directed repair (HDR) efficiency 9 to 31% (depending on cell cycle phase) and increases Cas9-mediated gene editing frequencies. |

Nocodazole Dilution Calculator

Nocodazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3187 mL | 16.5937 mL | 33.1873 mL | 66.3746 mL | 82.9683 mL |
| 5 mM | 0.6637 mL | 3.3187 mL | 6.6375 mL | 13.2749 mL | 16.5937 mL |
| 10 mM | 0.3319 mL | 1.6594 mL | 3.3187 mL | 6.6375 mL | 8.2968 mL |
| 50 mM | 0.0664 mL | 0.3319 mL | 0.6637 mL | 1.3275 mL | 1.6594 mL |
| 100 mM | 0.0332 mL | 0.1659 mL | 0.3319 mL | 0.6637 mL | 0.8297 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nocodazole is a reversible anti-neoplastic agent which exerts its effect in cells by interfering with the polymerization of microtubules (tubulin).
- Isotachioside
Catalog No.:BCN5230
CAS No.:31427-08-4
- IU1
Catalog No.:BCC2086
CAS No.:314245-33-5
- BPTES
Catalog No.:BCC6506
CAS No.:314045-39-1
- (R)-(-)-Apomorphine hydrochloride
Catalog No.:BCC7250
CAS No.:314-19-2
- Evans Blue tetrasodium salt
Catalog No.:BCC6815
CAS No.:314-13-6
- VDM 11
Catalog No.:BCC7044
CAS No.:313998-81-1
- [cPP1-7,NPY19-23,Ala31,Aib32,Gln34] - hPancreatic Polypeptide
Catalog No.:BCC5750
CAS No.:313988-89-5
- PU 02
Catalog No.:BCC6265
CAS No.:313984-77-9
- o-3M3FBS
Catalog No.:BCC7210
CAS No.:313981-55-4
- FLI-06
Catalog No.:BCC5110
CAS No.:313967-18-9
- [Des-octanoyl]-Ghrelin (human)
Catalog No.:BCC7304
CAS No.:313951-59-6
- 13-Oxo-9,11-octadecadienoic acid
Catalog No.:BCC8437
CAS No.:31385-09-8
- 4-Amino-3-nitrobenzophenone
Catalog No.:BCC8682
CAS No.:31431-19-3
- Mebendazole
Catalog No.:BCC9016
CAS No.:31431-39-7
- 6-Methoxysalicylic Acid
Catalog No.:BCC8288
CAS No.:3147-64-6
- Sunifiram
Catalog No.:BCC4167
CAS No.:314728-85-3
- Crotaline
Catalog No.:BCN4983
CAS No.:315-22-0
- Allopurinol
Catalog No.:BCC3720
CAS No.:315-30-0
- Testosterone enanthate
Catalog No.:BCC9169
CAS No.:315-37-7
- Acetylheliosupine
Catalog No.:BCN1981
CAS No.:31514-30-4
- PAC-1
Catalog No.:BCC3600
CAS No.:315183-21-2
- Ifflaiamine
Catalog No.:BCN7061
CAS No.:31520-95-3
- Sutherlandin trans-p-coumarate
Catalog No.:BCN5231
CAS No.:315236-68-1
- Isobavachin
Catalog No.:BCN5232
CAS No.:31524-62-6
An RNA-binding protein, RNP-1, protects microtubules from nocodazole and localizes to the leading edge during cytokinesis and cell migration in Dictyostelium cells.[Pubmed:27569394]
Acta Pharmacol Sin. 2016 Nov;37(11):1449-1457.
AIM: RNA-binding proteins are a large group of regulators (800-1000 in humans), some of which play significant roles in mRNA local translation. In this study, we analyzed the functions of the protein RNP-1, which was previously discovered in a genetic selection screen for Nocodazole suppression. METHODS: The growth rates and the microtubule networks of Dictyostelium cells were assessed with or without Nocodazole (10 mumol/L) in suspension culture. Fluorescent images of RNP-1-GFP and RFP-tubulin were captured when cells were undergoing cytokinesis, then the GFP signal intensity and distance to the nearest centrosome were analyzed by using a computer program written in Matlab((R)). The RNP-1-GFP-expresseding cells were polarized, and the time-lapse images of cells were captured when cells were chemotaxing to a cAMP source. RESULTS: Over-expression of RNP-1 rescued the growth defects caused by the microtubule-destabilizing agent Nocodazole. Over-expression of RNP-1 protected microtubules from Nocodazole treatment. In cells undergoing cytokinesis, the RNP-1 protein was localized to the polar regions of the cell cortex, and protein levels decreased proportionally as the power of the distance from the cell cortex to the nearest centrosome. In chemotactic cells, the RNP-1 protein localized to the leading edge of moving cells. Sequence analysis revealed that RNP-1 has two RNA-binding domains and is related to cytosolic poly(A)-binding proteins (PABPCs) in humans. CONCLUSION: RNP-1 has roles in protecting microtubules and in directing cortical movement during cytokinesis and cell migration in Dictyostelium cells. The sequence similarity of RNP-1 to human PABPCs suggests that PABPCs may have similar functions in mammalian cells, perhaps in regulating microtubule dynamics and functions during cortical movement in cytokinesis and cell migration.
Nocodazole treatment interrupted Brucella abortus invasion in RAW 264.7 cells, and successfully attenuated splenic proliferation with enhanced inflammatory response in mice.[Pubmed:28017899]
Microb Pathog. 2017 Feb;103:87-93.
Brucellosis is one of the most important and widespread zoonosis worldwide responsible for serious economic losses and considerable public health burden. In this study, we investigated the modulatory effect of a microtubule-inhibitor, Nocodazole, on B. abortus infection in murine macrophages and in a mouse model. Nocodazole activated macrophages and directly inhibited the growth of Brucella in a dose-dependent manner. Nocodazole increased adhesion but reduced invasion and intracellular growth of Brucella in macrophages although it did not affect co-localization of Brucella with LAMP-1. In addition, Nocodazole negatively affected actin polymerization, and weakly activated ERK and p38alpha but significantly activated JNK in non-infected cells. After subsequent infection, Nocodazole weakly inhibited activation of ERK and p38alpha. For the in vivo tests, Nocodazole -treated mice displayed elevated levels of IFN-gamma, MCP-1 and IL-10 while Brucella-infected Nocodazole -treated mice showed high levels of TNF, IFN-gamma, MCP-1, IL-10 and IL-6 as compared to controls. Furthermore, Nocodazole treatment reduced inflammation and Brucella proliferation in the spleens of mice. These findings highlight the potential use of Nocodazole for the control of brucellosis although further investigations are encouraged to validate its therapeutic use in animal hosts.
Nocodazole Induced Suicidal Death of Human Erythrocytes.[Pubmed:26824457]
Cell Physiol Biochem. 2016;38(1):379-92.
BACKGROUND: The microtubule assembly inhibitor Nocodazole has been shown to trigger caspase-independent mitotic death and caspase dependent apoptosis. Similar to apoptosis of nucleated cells, erythrocytes may undergo eryptosis, the suicidal erythrocyte death characterized by cell shrinkage and cell membrane scrambling with phosphatidylserine translocation to the erythrocyte surface. Stimulators of eryptosis include increase of cytosolic Ca2+ activity ([Ca2+]i), oxidative stress and ceramide. The present study explored, whether and how Nocodazole induces eryptosis. METHODS: Flow cytometry was employed to determine phosphatidylserine exposure at the cell surface from annexin-V-binding, cell volume from forward scatter, [Ca2+]i from Fluo3-fluorescence, the abundance of reactive oxygen species (ROS) from 2',7'-dichlorodihydrofluorescein (DCF) diacetate dependent fluorescence as well as ceramide surface abundance utilizing specific antibodies. Tubulin abundance was quantified by TubulinTracker Green reagent and visualized by confocal microscopy. RESULTS: A 48 hours exposure of human erythrocytes to Nocodazole (>/= 30 microg/ml) significantly increased the percentage of annexin-V-binding cells without significantly modifying average forward scatter. Nocodazole significantly increased Fluo3-fluorescence, significantly increased DCF fluorescence and significantly increased ceramide surface abundance. The effect of Nocodazole on annexin-V-binding was significantly blunted, but not abolished by removal of extracellular Ca2+ and was not modified in the presence of Caspase 3 inhibitor zVAD (1 microM). Nocodazole treatment reduced the content of total tubulin. CONCLUSIONS: Nocodazole triggers cell shrinkage and phospholipid scrambling of the erythrocyte cell membrane, an effect in part due to stimulation of Ca2+ entry, oxidative stress and ceramide.
Death-associated protein kinase 2 mediates nocodazole-induced apoptosis through interaction with tubulin.[Pubmed:26529546]
Biochem Biophys Res Commun. 2015 Dec 4-11;468(1-2):113-8.
Death-associated protein kinase 2 (DAPK2) is a positive regulator of apoptosis. Although we recently reported that 14-3-3 proteins inhibit DAPK2 activity and its subsequent apoptotic effects via binding to DAPK2, the molecular mechanisms underlying the DAPK2-mediated apoptotic pathway remain unclear. Therefore, we attempted to further identify DAPK2-interacting proteins using pull-down assays and mass spectrometry. The microtubule beta-tubulin was identified as a novel DAPK2-binding protein in HeLa cells. Pull-down assays revealed that DAPK2 interacted with the alpha/beta-tubulin heterodimer, and that the C-terminal region of DAPK2, which differs from that of other DAPK family members, was sufficient for the association with beta-tubulin. Although the microtubule-depolymerizing agent Nocodazole induced apoptosis in HeLa cells, the level of apoptosis was significantly decreased in the DAPK2 knockdown cells. Furthermore, we found that treatment with Nocodazole resulted in an increased binding of DAPK2 to beta-tubulin. These findings indicate that DAPK2 mediates Nocodazole-induced apoptosis via binding to tubulin.
Methods in mammalian autophagy research.[Pubmed:20144757]
Cell. 2010 Feb 5;140(3):313-26.
Autophagy has been implicated in many physiological and pathological processes. Accordingly, there is a growing scientific need to accurately identify, quantify, and manipulate the process of autophagy. However, as autophagy involves dynamic and complicated processes, it is often analyzed incorrectly. In this Primer, we discuss methods to monitor autophagy and to modulate autophagic activity, with a primary focus on mammalian macroautophagy.
Microtubule disruption inhibits autophagosome-lysosome fusion: implications for studying the roles of aggresomes in polyglutamine diseases.[Pubmed:15325591]
Int J Biochem Cell Biol. 2004 Dec;36(12):2541-50.
Large cytoplasmic inclusions called aggresomes are seen in many protein conformational diseases including Huntington's disease and Parkinson's disease. The roles of inclusions and aggresomes in these diseases are unresolved critical issues that have been vigorously debated. Two recent studies used microtubule disruption with Nocodazole to inhibit aggresome formation and observed increased toxicity of expanded polyglutamines in the context of huntingtin exon 1 and a truncated androgen receptor. Increased toxicity of expanded polyglutamines in the presence of Nocodazole was correlated with decreased protein turnover, leading the authors to conclude that aggresomes were cytoprotective and that they directly enhanced clearance of the toxic proteins. Here we show that Nocodazole has additional effects, which provide a simple alternative explanation for these previous observations. We confirmed aggresome formation in cells expressing proteins with polyalanine and polyglutamine expansions. As expected, we found a reduction in aggresome formation when microtubule function was disrupted using Nocodazole. However, in addition to this effect, Nocodazole treatment increased the proportions of cells with nuclear inclusions in PC12 cells expressing huntingtin exon 1 with 74 glutamines. This can be explained as Nocodazole inhibits autophagosome-lysosome fusion, a key step in mutant huntingtin exon 1 clearance. This effect alone can explain the previous observations with this compound in polyglutamine diseases and raises doubts about the interpretation of some of the data that have been used to argue that aggresomes protect against polyglutamine mutations.
Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro.[Pubmed:9201709]
Mol Biol Cell. 1997 Jun;8(6):973-85.
Previous studies demonstrated that nanomolar concentrations of Nocodazole can block cells in mitosis without net microtubule disassembly and resulted in the hypothesis that this block was due to a Nocodazole-induced stabilization of microtubules. We tested this hypothesis by examining the effects of nanomolar concentrations of Nocodazole on microtubule dynamic instability in interphase cells and in vitro with purified brain tubulin. Newt lung epithelial cell microtubules were visualized by video-enhanced differential interference contrast microscopy and cells were perfused with solutions of Nocodazole ranging in concentration from 4 to 400 nM. Microtubules showed a loss of the two-state behavior typical of dynamic instability as evidenced by the addition of a third state where they exhibited little net change in length (a paused state). Nocodazole perfusion also resulted in slower elongation and shortening velocities, increased catastrophe, and an overall decrease in microtubule turnover. Experiments performed on BSC-1 cells that were microinjected with rhodamine-labeled tubulin, incubated in Nocodazole for 1 h, and visualized by using low-light-level fluorescence microscopy showed similar results except that Nocodazole-treated BSC-1 cells showed a decrease in catastrophe. To gain insight into possible mechanisms responsible for changes in dynamic instability, we examined the effects of 4 nM to 12 microM Nocodazole on the assembly of purified tubulin from axoneme seeds. At both microtubule plus and minus ends, perfusion with Nocodazole resulted in a dose-dependent decrease in elongation and shortening velocities, increase in pause duration and catastrophe frequency, and decrease in rescue frequency. These effects, which result in an overall decrease in microtubule turnover after Nocodazole treatment, suggest that the mitotic block observed is due to a reduction in microtubule dynamic turnover. In addition, the in vitro results are similar to the effects of increasing concentrations of GDP-tubulin (TuD) subunits on microtubule assembly. Given that Nocodazole increases tubulin GTPase activity, we propose that Nocodazole acts by generating TuD subunits that then alter dynamic instability.


