IsotachiosideCAS# 31427-08-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 31427-08-4 | SDF | Download SDF |
| PubChem ID | 15098566 | Appearance | Powder |
| Formula | C13H18O8 | M.Wt | 302.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
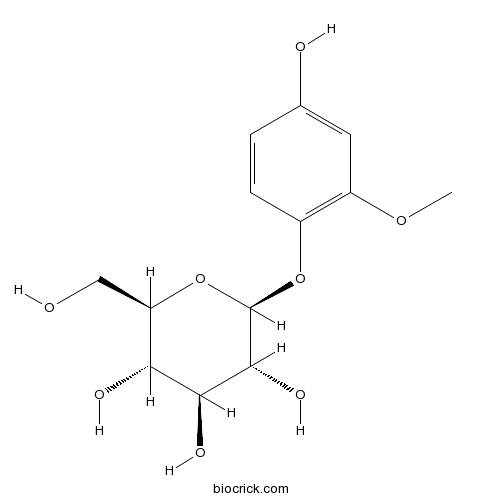
| Chemical Name | (2S,3R,4S,5S,6R)-2-(4-hydroxy-2-methoxyphenoxy)-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | COC1=C(C=CC(=C1)O)OC2C(C(C(C(O2)CO)O)O)O | ||
| Standard InChIKey | LWEHRPZXRYZMDC-UJPOAAIJSA-N | ||
| Standard InChI | InChI=1S/C13H18O8/c1-19-8-4-6(15)2-3-7(8)20-13-12(18)11(17)10(16)9(5-14)21-13/h2-4,9-18H,5H2,1H3/t9-,10-,11+,12-,13-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Isotachioside has15-lipoxygenase (15-LO) inhibitory activities. |
| Targets | LOX |

Isotachioside Dilution Calculator

Isotachioside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.308 mL | 16.5399 mL | 33.0797 mL | 66.1594 mL | 82.6993 mL |
| 5 mM | 0.6616 mL | 3.308 mL | 6.6159 mL | 13.2319 mL | 16.5399 mL |
| 10 mM | 0.3308 mL | 1.654 mL | 3.308 mL | 6.6159 mL | 8.2699 mL |
| 50 mM | 0.0662 mL | 0.3308 mL | 0.6616 mL | 1.3232 mL | 1.654 mL |
| 100 mM | 0.0331 mL | 0.1654 mL | 0.3308 mL | 0.6616 mL | 0.827 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- IU1
Catalog No.:BCC2086
CAS No.:314245-33-5
- BPTES
Catalog No.:BCC6506
CAS No.:314045-39-1
- (R)-(-)-Apomorphine hydrochloride
Catalog No.:BCC7250
CAS No.:314-19-2
- Evans Blue tetrasodium salt
Catalog No.:BCC6815
CAS No.:314-13-6
- VDM 11
Catalog No.:BCC7044
CAS No.:313998-81-1
- [cPP1-7,NPY19-23,Ala31,Aib32,Gln34] - hPancreatic Polypeptide
Catalog No.:BCC5750
CAS No.:313988-89-5
- PU 02
Catalog No.:BCC6265
CAS No.:313984-77-9
- o-3M3FBS
Catalog No.:BCC7210
CAS No.:313981-55-4
- FLI-06
Catalog No.:BCC5110
CAS No.:313967-18-9
- [Des-octanoyl]-Ghrelin (human)
Catalog No.:BCC7304
CAS No.:313951-59-6
- 13-Oxo-9,11-octadecadienoic acid
Catalog No.:BCC8437
CAS No.:31385-09-8
- PD 118057
Catalog No.:BCC7499
CAS No.:313674-97-4
- Nocodazole
Catalog No.:BCC3826
CAS No.:31430-18-9
- 4-Amino-3-nitrobenzophenone
Catalog No.:BCC8682
CAS No.:31431-19-3
- Mebendazole
Catalog No.:BCC9016
CAS No.:31431-39-7
- 6-Methoxysalicylic Acid
Catalog No.:BCC8288
CAS No.:3147-64-6
- Sunifiram
Catalog No.:BCC4167
CAS No.:314728-85-3
- Crotaline
Catalog No.:BCN4983
CAS No.:315-22-0
- Allopurinol
Catalog No.:BCC3720
CAS No.:315-30-0
- Testosterone enanthate
Catalog No.:BCC9169
CAS No.:315-37-7
- Acetylheliosupine
Catalog No.:BCN1981
CAS No.:31514-30-4
- PAC-1
Catalog No.:BCC3600
CAS No.:315183-21-2
- Ifflaiamine
Catalog No.:BCN7061
CAS No.:31520-95-3
- Sutherlandin trans-p-coumarate
Catalog No.:BCN5231
CAS No.:315236-68-1
Antioxidant and 15-lipoxygenase inhibitory activity of rotenoids, isoflavones and phenolic glycosides from Sarcolobus globosus.[Pubmed:16701962]
Fitoterapia. 2006 Jun;77(4):290-5.
From Sarcolobus globosus, two rotenoids (villosinol and 6-oxo-6a,12a-dehydrodeguelin), one isoflavone (genistin) and four phenolic glycosides (vanillic acid 4-O-beta-d-glucoside, glucosyringic acid, tachioside and Isotachioside) were identified for the first time from this species. Extracts and compounds from S. globosus were evaluated for their DPPH radical scavenging and 15-lipoxygenase (15-LO) inhibitory activities. All tested rotenoids were found to inhibit 15-LO, while they lacked DPPH radical scavenging effect.
[Chemical constituents from Hydrangea paniculata].[Pubmed:21355271]
Zhongguo Zhong Yao Za Zhi. 2010 Nov;35(22):3007-9.
In order to study the chemical constituents of the plant of Hydrangea paniculata and provide reference for the study of the bioactive substances, we isolated nine compounds from the dried branches of H. paniculata. Their structures were determined by application of spectroscopic (NMR, MS) and chemical methods. These compounds were identified as skimmin (1), Isotachioside (2), 8-methoxy-7-O-beta-D-glucopyranosyloxy coumarin glycoside (3), scopolin (4), 1-(alpha-L-rhamnosyl-(1 --> 6) -O-beta-D-glucopyranosyloxy) - 3, 4, 5-trimethoxybenzene (5), apiosylskimmin (6), umbelliferone (7), scopoletin (8), 7-hydroxy-8-methoxycoumarin (9). Compounds 1 - 7 were isolated from H. paniculata for the first time.
Polygonophenone, the first MEM-substituted natural product, from Polygonum maritimum.[Pubmed:19199482]
J Nat Prod. 2009 Feb 27;72(2):187-9.
An unprecedented natural acetophenone, polygonophenone (1), and a new resorcinol, polygonocinol (2), were isolated from the dichloromethane and methanol extracts of Polygonum maritimum and identified as 2-hydroxy-4-[(2-methoxyethoxy)methoxy] acetophenone and 2-methyl-5-nonadecylresorcinol, respectively. In addition, 11 known compounds were identified, namely, the sesquiterpenoid (+)-8-hydroxycalamene, four ferulic acid esters (tetracosyl, hexacosyl, octacosyl, and triacontyl ferulate), the arylpropane broussonin B, the flavonoids quercetin, quercitrin, and (+)-catechin, the hydroquinone glucoside Isotachioside, and beta-sitosterol. The structures of the new compounds were elucidated on the basis of their NMR and MS data. It is noteworthy that polygonophenone (1) is the first naturally methoxyethoxymethyl (MEM)-substituted natural product, and its isolation gives support for the use of MEM protection in biomimetic synthetic schemes.
Phenolic glycosides from Lindera obtusiloba and their anti-allergic inflammatory activities.[Pubmed:23513723]
Nat Prod Commun. 2013 Feb;8(2):181-2.
Eight phenolic glycosides, tachioside (1), Isotachioside (2), koaburaside (3), 2,6-dimethoxy-4-hydroxyphenyl-1-O-beta-D-glucopyranoside (4), 4,6-dihydroxy-2-methoxyphenyl-1-O-beta-D-glucopyranoside (5), a mixture of erigeside C (6a) and salidroside (6b), and 6-hydroxyphenyl)-1-O-beta-D-glucopyranoside (7) were isolated from the stems of Lindera obtusiloba Blume. The structures of the isolates were determined by 1H-, 13C-NMR, COSY, HMQC, and HMBC spectroscopy. To evaluate their anti-allergic inflammatory activities, the inhibitory effects of isolates (1-7) on histamine release and on the gene expressions of tumor necrosis factor (TNF)-a and interleukin (IL)-6 were examined using human mast cells; previous studies have reported that TNF-alpha and IL-6 release from mast cells is positively related to the severity of allergic symptoms. Of the tested compounds, koaburaside (3), 2,6-dimethoxy-4-hydroxyphenyl-1-O-beta-D-glucopyranoside (4), and (6-hydroxyphenyl)-1-O-beta-D-glucopyranoside (7) suppressed histamine release from mast cells as compared with gallic acid (positive control). In particular, 6-hydroxyphenyl)-1-O-beta-D-glucopyranoside (7) attenuated the gene expressions of the proinflammatory cytokines TNF-alpha and IL-6 in human mast cells. Our results support the notion that phenolic glycosides isolated from L. obtusiloba inhibit mast-cell-derived allergic inflammation, histamine, and proinflammatory cytokines.


