PD 118057Selective KV11.1 (hERG) channel activator CAS# 313674-97-4 |
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- R547
Catalog No.:BCC3927
CAS No.:741713-40-6
- Palbociclib (PD0332991) Isethionate
Catalog No.:BCC3698
CAS No.:827022-33-3
- AT7519
Catalog No.:BCC2541
CAS No.:844442-38-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 313674-97-4 | SDF | Download SDF |
| PubChem ID | 9864959 | Appearance | Powder |
| Formula | C21H17Cl2NO2 | M.Wt | 386.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 20 mM in ethanol | ||
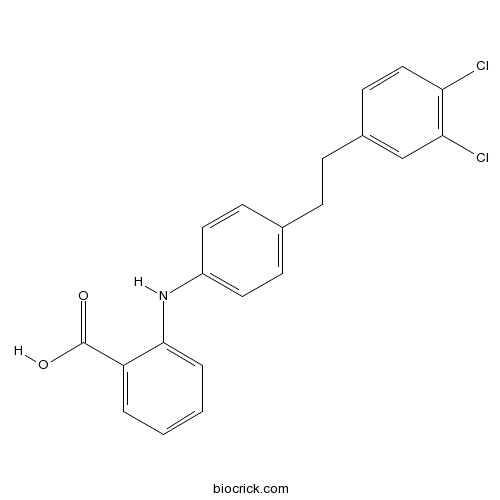
| Chemical Name | 2-[4-[2-(3,4-dichlorophenyl)ethyl]anilino]benzoic acid | ||
| SMILES | C1=CC=C(C(=C1)C(=O)O)NC2=CC=C(C=C2)CCC3=CC(=C(C=C3)Cl)Cl | ||
| Standard InChIKey | ZCQOSCDABPVAFB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H17Cl2NO2/c22-18-12-9-15(13-19(18)23)6-5-14-7-10-16(11-8-14)24-20-4-2-1-3-17(20)21(25)26/h1-4,7-13,24H,5-6H2,(H,25,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Human ether-a-go-go (hERG) KV11.1 potassium channel activator. Displays no major effect on INa, ICa/L, IK1 and IKs currents. Causes resting membrane hyperpolarization and decreases action potential duration in rat myocytes in vitro. |

PD 118057 Dilution Calculator

PD 118057 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5889 mL | 12.9443 mL | 25.8886 mL | 51.7773 mL | 64.7216 mL |
| 5 mM | 0.5178 mL | 2.5889 mL | 5.1777 mL | 10.3555 mL | 12.9443 mL |
| 10 mM | 0.2589 mL | 1.2944 mL | 2.5889 mL | 5.1777 mL | 6.4722 mL |
| 50 mM | 0.0518 mL | 0.2589 mL | 0.5178 mL | 1.0355 mL | 1.2944 mL |
| 100 mM | 0.0259 mL | 0.1294 mL | 0.2589 mL | 0.5178 mL | 0.6472 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bombesin
Catalog No.:BCC5708
CAS No.:31362-50-2
- INH1
Catalog No.:BCC6040
CAS No.:313553-47-8
- T0070907
Catalog No.:BCC2261
CAS No.:313516-66-4
- VU 590 dihydrochloride
Catalog No.:BCC7803
CAS No.:313505-85-0
- Reversan
Catalog No.:BCC7764
CAS No.:313397-13-6
- Regadenoson
Catalog No.:BCC6438
CAS No.:313348-27-5
- ICA 121431
Catalog No.:BCC6358
CAS No.:313254-51-2
- Aristolochic acid A
Catalog No.:BCN6262
CAS No.:313-67-7
- Estradiol Cypionate
Catalog No.:BCC4477
CAS No.:313-06-4
- Arjunic acid
Catalog No.:BCN5229
CAS No.:31298-06-3
- LDN-27219
Catalog No.:BCC6236
CAS No.:312946-37-5
- TCS JNK 5a
Catalog No.:BCC5148
CAS No.:312917-14-9
- 13-Oxo-9,11-octadecadienoic acid
Catalog No.:BCC8437
CAS No.:31385-09-8
- [Des-octanoyl]-Ghrelin (human)
Catalog No.:BCC7304
CAS No.:313951-59-6
- FLI-06
Catalog No.:BCC5110
CAS No.:313967-18-9
- o-3M3FBS
Catalog No.:BCC7210
CAS No.:313981-55-4
- PU 02
Catalog No.:BCC6265
CAS No.:313984-77-9
- [cPP1-7,NPY19-23,Ala31,Aib32,Gln34] - hPancreatic Polypeptide
Catalog No.:BCC5750
CAS No.:313988-89-5
- VDM 11
Catalog No.:BCC7044
CAS No.:313998-81-1
- Evans Blue tetrasodium salt
Catalog No.:BCC6815
CAS No.:314-13-6
- (R)-(-)-Apomorphine hydrochloride
Catalog No.:BCC7250
CAS No.:314-19-2
- BPTES
Catalog No.:BCC6506
CAS No.:314045-39-1
- IU1
Catalog No.:BCC2086
CAS No.:314245-33-5
- Isotachioside
Catalog No.:BCN5230
CAS No.:31427-08-4
Pharmacologic Approach to Defective Protein Trafficking in the E637K-hERG Mutant with PD-118057 and Thapsigargin.[Pubmed:23840331]
PLoS One. 2013 Jun 19;8(6):e65481.
BACKGROUND: Treatment of LQT2 is inadequate. Many drugs which can pharmacologically rescue defective protein trafficking in LQT2 also result in potent blockade of HERG current, negating their therapeutic benefit. It is reported that PD-118057 and thapsigargin can rescue LQT2 without hERG channel blockade, but the precise mechanism of action is unknown. Furthermore, the effect of PD-118057 and thapsigargin on the dominant negative E637K-hERG mutant has not been previously investigated. OBJECTIVE: IN THIS STUDY, WE INVESTIGATED: (a) the effect of PD-118057 and thapsigargin on the current amplitudes of WT-hERG and WT/E637K-hERG channels; (b) the effect of PD-118057 and thapsigargin on the biophysical properties of WT-hERG and WT/E637K-hERG channels; (c) whether drug treatment can rescue channel processing and trafficking defects of the WT/E637K-hERG mutant. METHODS: The whole-cell Patch-clamp technique was used to assess the effect of PD-118057 and thapsigargin on the electrophysiological characteristics of the rapidly activating delayed rectifier K(+) current (Ikr) of the hERG protein channel. Western blot was done to investigate pharmacological rescue on hERG protein channel function. RESULTS: In our study, PD-118057 was shown to significantly enhance both the maximum current amplitude and tail current amplitude, but did not alter the gating and kinetic properties of the WT-hERG channel, with the exception of accelerating steady-state inactivation. Additionally, thapsigargin shows a similar result as PD-118057 for the WT-hERG channel, but with the exception of attenuating steady-state inactivation. However, for the WT/E637K-hERG channel, PD-118057 had no effect on either the current or on the gating and kinetic properties. Furthermore, thapsigargin treatment did not alter the current or the gating and kinetic properties of the WT/E637K-hERG channel, with the exception of opening at more positive voltages. CONCLUSION: Our findings illustrate that neither PD-118057 nor thapsigargin play a role in correcting the dominant-negative effect of the E637K-hERG mutant.
PD-118057 contacts the pore helix of hERG1 channels to attenuate inactivation and enhance K+ conductance.[Pubmed:19892732]
Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):20075-80.
Human ether-a-go-go-related gene 1 (hERG1) K(+) channels mediate repolarization of cardiac action potentials. Unintended block of hERG1 channels by some drugs can prolong the QT interval and induce arrhythmia. Recently, hERG1 channel agonists were discovered and, based on their mechanisms of action can be classified into two types. RPR260243 [(3R,4R)-4-[3-(6-methoxy-quinolin-4-yl)-3-oxo-propyl]-1-[3-(2,3,5 trifluorophenyl)-prop-2-ynyl]-piperidine-3-carboxylic acid], a type 1 agonist, binds to residues located near the intracellular end of S5 and S6 transmembrane segments and activates hERG1 channels by a dual mechanism of slowed deactivation and attenuated P-type inactivation. As defined here, type 2 agonists such as PD-118057 [2-(4-[2-(3,4-dichloro-phenyl)-ethyl]-phenylamino)-benzoic acid] attenuate inactivation but do not slow deactivation. At 10 muM, PD-118057 shifted the half-point for inactivation of wild-type hERG1 channels by +19 mV and increased peak outward current by 136%. Scanning mutagenesis and functional characterization of 44 mutant channels expressed in Xenopus oocytes was used to identify the major structural determinants of the binding site for PD-118057. Single mutations of residues in the pore helix (F619) or the S6 segment (L646) of hERG1 eliminated agonist activity. Mutation of a nearby residues in the S6 segment (C643, M645) enhanced drug activity, presumably by reducing steric hindrance for drug binding. Molecular modeling indicates that PD-118057 binds to a hydrophobic pocket formed by L646 of one hERG1 subunit and F619 of an adjacent subunit. We conclude that direct interaction of PD-118057 with the pore helix attenuates fast P-type inactivation and increases open probability of hERG1 channels.
Pharmacological and biophysical isolation of K+ currents encoded by ether-a-go-go-related genes in murine hepatic portal vein smooth muscle cells.[Pubmed:16870833]
Am J Physiol Cell Physiol. 2007 Jan;292(1):C468-76.
Previous studies have shown that murine portal vein myocytes express ether-a-go-go related genes (ERGs) and exhibit distinctive currents when recorded under symmetrical K(+) conditions. The aim of the present study was to characterize ERG channel currents evoked from a negative holding potential under conditions more pertinent to a physiological scenario to assess the possible functional impact of this conductance. Currents were recorded with ruptured or perforated patch variants of the whole cell technique from a holding potential of -60 mV. Application of three structurally distinct and selective ERG channel blockers, E-4031, dofetilide, and the peptide toxin BeKM-1, all inhibited a significant proportion of the outward current and abolished inward currents with distinctive "hooked" kinetics recorded on repolarization. Dofetilide-sensitive currents at negative potentials evoked by depolarization to +40 mV had a voltage-dependent time to peak and rate of decay characteristic of ERG channels. Application of the novel ERG channel activator PD-118057 (1-10 microM) markedly enhanced the hooked inward currents evoked by membrane depolarization and hyperpolarized the resting membrane potential recorded by current clamp and the perforated patch configuration by approximately 20 mV. In contrast, ERG channel blockade by dofetilide (1 microM) depolarized the resting membrane potential by approximately 8 mV. These data are the first record of ERG channel currents in smooth muscle cells under quasi-physiological conditions that suggest that ERG channels contribute to the resting membrane potential in these cells.
Novel potent human ether-a-go-go-related gene (hERG) potassium channel enhancers and their in vitro antiarrhythmic activity.[Pubmed:15976038]
Mol Pharmacol. 2005 Sep;68(3):876-84.
A variety of drugs has been reported to cause acquired long QT syndrome through inhibition of the IKr channel. Screening compounds in early discovery and development stages against their ability to inhibit IKr or the hERG channel has therefore become an indispensable procedure in the pharmaceutical industry. In contrast to numerous hERG channel blockers discovered during screening, only (3R,4R)-4-[3-(6-methoxyquinolin-4-yl)-3-oxo-propyl]-1-[3-(2,3,5-trifluoro-phenyl) -prop-2-ynyl]-piperidine-3-carboxylic acid (RPR260243) has been reported so far to enhance the hERG current. In this article, we describe several potent mechanistically distinct hERG channel enhancers. One example is PD-118057 (2-{4-[2-(3,4-dichloro-phenyl)-ethyl]-phenylamino}-benzoic acid) which produced average increases of 5.5 +/- 1.1, 44.8 +/- 3.1, and 111.1 +/- 21.7% in the peak tail hERG current at 1, 3, and 10 muM, respectively, in human embryonic kidney 293 cells. PD-118057 did not affect the voltage dependence and kinetics of gating parameters, nor did it require open conformation of the channel. In isolated guinea pig cardiomyocytes, PD-118057 showed no major effect on I(Na), I(Ca,L), I(K1), and I(Ks). PD-118057 shortened the action potential duration and QT interval in arterially perfused rabbit ventricular wedge preparation in a concentration-dependent manner. The presence of 3 muM PD-118057 prevented action potential duration and QT prolongation caused by dofetilide. "Early after-depolarizations" induced by dofetilide were also completely eliminated by 3 microM PD-118057. Although further investigation is warranted to evaluate the therapeutic value and safety profile of these compounds, our data support the notion that hERG activation by pharmaceuticals may offer a new approach in the treatment of delayed repolarization conditions, which may occur in patients with inherited or acquired long QT syndrome, congestive heart failure, and diabetes.


