PU 02Negative allosteric modulator of 5-HT3 CAS# 313984-77-9 |
- VX-661
Catalog No.:BCC1241
CAS No.:1152311-62-0
- IOWH-032
Catalog No.:BCC3922
CAS No.:1191252-49-9
- CFTRinh-172
Catalog No.:BCC4419
CAS No.:307510-92-5
- GlyH-101
Catalog No.:BCC4104
CAS No.:328541-79-3
- Ivacaftor (VX-770)
Catalog No.:BCC2478
CAS No.:873054-44-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 313984-77-9 | SDF | Download SDF |
| PubChem ID | 720937 | Appearance | Powder |
| Formula | C16H12N4S | M.Wt | 292.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
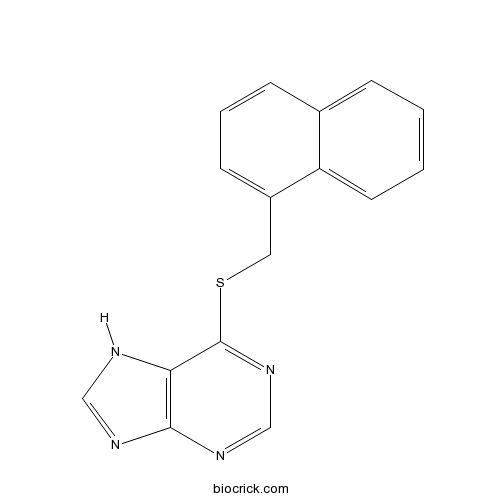
| Chemical Name | 6-(naphthalen-1-ylmethylsulfanyl)-7H-purine | ||
| SMILES | C1=CC=C2C(=C1)C=CC=C2CSC3=NC=NC4=C3NC=N4 | ||
| Standard InChIKey | BGMSTNYJYPSLHN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12N4S/c1-2-7-13-11(4-1)5-3-6-12(13)8-21-16-14-15(18-9-17-14)19-10-20-16/h1-7,9-10H,8H2,(H,17,18,19,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Negative allosteric modulator of 5-HT3 receptors (IC50 values are 0.36 and 0.73 μM in HEK293 cells transfected with human 5-HT3A and 5-HT3AB receptors respectively). |

PU 02 Dilution Calculator

PU 02 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4204 mL | 17.1022 mL | 34.2044 mL | 68.4088 mL | 85.511 mL |
| 5 mM | 0.6841 mL | 3.4204 mL | 6.8409 mL | 13.6818 mL | 17.1022 mL |
| 10 mM | 0.342 mL | 1.7102 mL | 3.4204 mL | 6.8409 mL | 8.5511 mL |
| 50 mM | 0.0684 mL | 0.342 mL | 0.6841 mL | 1.3682 mL | 1.7102 mL |
| 100 mM | 0.0342 mL | 0.171 mL | 0.342 mL | 0.6841 mL | 0.8551 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- o-3M3FBS
Catalog No.:BCC7210
CAS No.:313981-55-4
- FLI-06
Catalog No.:BCC5110
CAS No.:313967-18-9
- [Des-octanoyl]-Ghrelin (human)
Catalog No.:BCC7304
CAS No.:313951-59-6
- 13-Oxo-9,11-octadecadienoic acid
Catalog No.:BCC8437
CAS No.:31385-09-8
- PD 118057
Catalog No.:BCC7499
CAS No.:313674-97-4
- Bombesin
Catalog No.:BCC5708
CAS No.:31362-50-2
- INH1
Catalog No.:BCC6040
CAS No.:313553-47-8
- T0070907
Catalog No.:BCC2261
CAS No.:313516-66-4
- VU 590 dihydrochloride
Catalog No.:BCC7803
CAS No.:313505-85-0
- Reversan
Catalog No.:BCC7764
CAS No.:313397-13-6
- Regadenoson
Catalog No.:BCC6438
CAS No.:313348-27-5
- ICA 121431
Catalog No.:BCC6358
CAS No.:313254-51-2
- [cPP1-7,NPY19-23,Ala31,Aib32,Gln34] - hPancreatic Polypeptide
Catalog No.:BCC5750
CAS No.:313988-89-5
- VDM 11
Catalog No.:BCC7044
CAS No.:313998-81-1
- Evans Blue tetrasodium salt
Catalog No.:BCC6815
CAS No.:314-13-6
- (R)-(-)-Apomorphine hydrochloride
Catalog No.:BCC7250
CAS No.:314-19-2
- BPTES
Catalog No.:BCC6506
CAS No.:314045-39-1
- IU1
Catalog No.:BCC2086
CAS No.:314245-33-5
- Isotachioside
Catalog No.:BCN5230
CAS No.:31427-08-4
- Nocodazole
Catalog No.:BCC3826
CAS No.:31430-18-9
- 4-Amino-3-nitrobenzophenone
Catalog No.:BCC8682
CAS No.:31431-19-3
- Mebendazole
Catalog No.:BCC9016
CAS No.:31431-39-7
- 6-Methoxysalicylic Acid
Catalog No.:BCC8288
CAS No.:3147-64-6
- Sunifiram
Catalog No.:BCC4167
CAS No.:314728-85-3
Discovery of a novel allosteric modulator of 5-HT3 receptors: inhibition and potentiation of Cys-loop receptor signaling through a conserved transmembrane intersubunit site.[Pubmed:22589534]
J Biol Chem. 2012 Jul 20;287(30):25241-54.
The ligand-gated ion channels in the Cys-loop receptor superfamily mediate the effects of neurotransmitters acetylcholine, serotonin, GABA, and glycine. Cys-loop receptor signaling is susceptible to modulation by ligands acting through numerous allosteric sites. Here we report the discovery of a novel class of negative allosteric modulators of the 5-HT(3) receptors (5-HT(3)Rs). PU02 (6-[(1-naphthylmethyl)thio]-9H-purine) is a potent and selective antagonist displaying IC(50) values of ~1 muM at 5-HT(3)Rs and substantially lower activities at other Cys-loop receptors. In an elaborate mutagenesis study of the 5-HT(3)A receptor guided by a homology model, PU02 is demonstrated to act through a transmembrane intersubunit site situated in the upper three helical turns of TM2 and TM3 in the (+)-subunit and TM1 and TM2 in the (-)-subunit. The Ser(248), Leu(288), Ile(290), Thr(294), and Gly(306) residues are identified as important molecular determinants of PU02 activity with minor contributions from Ser(292) and Val(310), and we propose that the naphthalene group of PU02 docks into the hydrophobic cavity formed by these. Interestingly, specific mutations of Ser(248), Thr(294), and Gly(306) convert PU02 into a complex modulator, potentiating and inhibiting 5-HT-evoked signaling through these mutants at low and high concentrations, respectively. The PU02 binding site in the 5-HT(3)R corresponds to allosteric sites in anionic Cys-loop receptors, which emphasizes the uniform nature of the molecular events underlying signaling through the receptors. Moreover, the dramatic changes in the functional properties of PU02 induced by subtle changes in its binding site bear witness to the delicate structural discrimination between allosteric inhibition and potentiation of Cys-loop receptors.


