CFTRinh-172CFTR inhibitor, highly potent and selective CAS# 307510-92-5 |
- Biperiden HCl
Catalog No.:BCC4565
CAS No.:1235-82-1
- Darifenacin
Catalog No.:BCC1516
CAS No.:133099-04-4
- Darifenacin HBr
Catalog No.:BCC4567
CAS No.:133099-07-7
- Cevimeline hydrochloride hemihydrate
Catalog No.:BCC1471
CAS No.:153504-70-2
- Umeclidinium bromide
Catalog No.:BCC2022
CAS No.:869113-09-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 307510-92-5 | SDF | Download SDF |
| PubChem ID | 1554208 | Appearance | Powder |
| Formula | C18H10F3NO3S2 | M.Wt | 409.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CFTR Inhibitor-172; CFTRinh-172 | ||
| Solubility | DMSO : 50 mg/mL (122.13 mM; Need ultrasonic) | ||
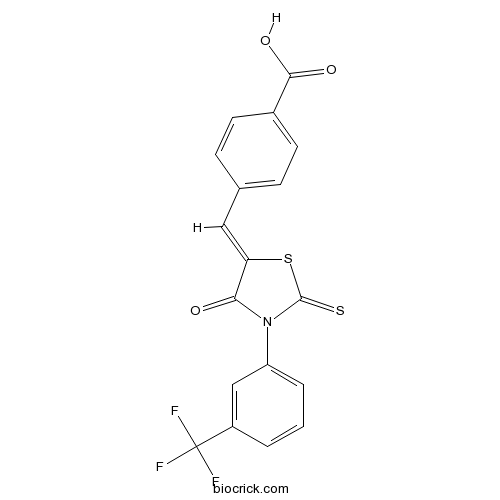
| Chemical Name | 4-[(Z)-[4-oxo-2-sulfanylidene-3-[3-(trifluoromethyl)phenyl]-1,3-thiazolidin-5-ylidene]methyl]benzoic acid | ||
| SMILES | C1=CC(=CC(=C1)N2C(=O)C(=CC3=CC=C(C=C3)C(=O)O)SC2=S)C(F)(F)F | ||
| Standard InChIKey | JIMHYXZZCWVCMI-ZSOIEALJSA-N | ||
| Standard InChI | InChI=1S/C18H10F3NO3S2/c19-18(20,21)12-2-1-3-13(9-12)22-15(23)14(27-17(22)26)8-10-4-6-11(7-5-10)16(24)25/h1-9H,(H,24,25)/b14-8- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Voltage-independent, selective CFTR chloride channel blocker (Ki = 300 nM) that alters channel gating. Blocks intestinal fluid secretion induced by cholera toxin and Escherichia coli and suppresses cyst growth in animal models of polycystic kidney disease. Orally active. Inhibits mitochondrial respiration and increases reactive oxygen species (ROS) production independently of CFTR in several cell lines. |

CFTRinh-172 Dilution Calculator

CFTRinh-172 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4426 mL | 12.213 mL | 24.426 mL | 48.852 mL | 61.065 mL |
| 5 mM | 0.4885 mL | 2.4426 mL | 4.8852 mL | 9.7704 mL | 12.213 mL |
| 10 mM | 0.2443 mL | 1.2213 mL | 2.4426 mL | 4.8852 mL | 6.1065 mL |
| 50 mM | 0.0489 mL | 0.2443 mL | 0.4885 mL | 0.977 mL | 1.2213 mL |
| 100 mM | 0.0244 mL | 0.1221 mL | 0.2443 mL | 0.4885 mL | 0.6106 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ki: 0.3 to 5 μM for channel opening
The cystic fibrosis (CF) transmembrane regulator (CFTR) is a cAMP-activated Cl channel expressed in epithelial cells of the lung, intestine, pancreas, and other tissues, where it facilitates transepithelial fluid transport. CFTRinh-172 is a highly potent and selective CFTR inhibitor.
In vitro: CFTRinh-172 could reversibly inhibit CFTR short-circuit current in less than 2 minutes in a voltage-independent manner. Moreover, at concentrations fully inhibiting CFTR, CFTRinh-172 did not prevent elevation of cellular cAMP or inhibit non-CFTR Cl–channels, multidrug resistance protein-1, ATP-sensitive K+ channels, or a series of other transporters [2].
In vivo: A single ip injection of CFTRinh-172 (250 μg/kg) in mice reduced by more than 90% cholera toxin–induced fluid secretion in the small intestine over 6 hours. CFTRinh-172 may be useful in developing large-animal models of cystic fibrosis and reducing intestinal fluid loss in cholera and other secretory diarrheas [3].
Clinical trial: Up to now, CFTRinh-172 is still in the preclinical development stage.
Reference:
[1] Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002 Dec;110(11):1651-8.
- Troxipide
Catalog No.:BCC4744
CAS No.:30751-05-4
- 1,4-Bis[2-(4-methyl-5-phenyloxazolyl)]benzene
Catalog No.:BCC8425
CAS No.:3073-87-8
- WAY 170523
Catalog No.:BCC2380
CAS No.:307002-73-9
- CL 82198 hydrochloride
Catalog No.:BCC2372
CAS No.:307002-71-7
- GW-1100
Catalog No.:BCC1610
CAS No.:306974-70-9
- IBC 293
Catalog No.:BCC7376
CAS No.:306935-41-1
- TCID
Catalog No.:BCC4449
CAS No.:30675-13-9
- 5-Methoxy-7-hydroxycoumarin
Catalog No.:BCN3538
CAS No.:3067-10-5
- Cycloolivil
Catalog No.:BCN4081
CAS No.:3064-05-9
- (20S)-Protopanaxdiol
Catalog No.:BCN1254
CAS No.:30636-90-9
- AVE 0991 sodium salt
Catalog No.:BCC4222
CAS No.:306288-04-0
- 2,2-Diphenylglycine
Catalog No.:BCC8496
CAS No.:3060-50-2
- SMER 28
Catalog No.:BCC7908
CAS No.:307538-42-7
- STF 083010
Catalog No.:BCC6209
CAS No.:307543-71-1
- Crosemperine
Catalog No.:BCN2074
CAS No.:30785-56-9
- [Des-octanoyl]-Ghrelin (rat)
Catalog No.:BCC5953
CAS No.:307950-60-3
- beta-Anhydrouzarigenin
Catalog No.:BCN5222
CAS No.:3080-20-4
- L-Theanine
Catalog No.:BCN2571
CAS No.:3081-61-6
- AEBSF.HCl
Catalog No.:BCC1219
CAS No.:30827-99-7
- 4-Oxododecanedioic acid
Catalog No.:BCN5223
CAS No.:30828-09-2
- Spongouridine
Catalog No.:BCC9152
CAS No.:3083-77-0
- PM00104
Catalog No.:BCC4237
CAS No.:308359-57-1
- Aloesin
Catalog No.:BCN8437
CAS No.:30861-27-9
- Kauran-18-Olc Acid,16,1719-Tnhydroxy-,(4A)
Catalog No.:BCC9235
CAS No.:308821-59-2
Revisiting CFTR inhibition: a comparative study of CFTRinh -172 and GlyH-101 inhibitors.[Pubmed:24758416]
Br J Pharmacol. 2014 Aug;171(15):3716-27.
BACKGROUND AND PURPOSE: For decades, inhibitors of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel have been used as tools to investigate the role and function of CFTR conductance in cystic fibrosis research. In the early 2000s, two new and potent inhibitors of CFTR, CFTRinh -172 and GlyH-101, were described and are now widely used to inhibit specifically CFTR. However, despite some evidence, the effects of both drugs on other types of Cl(-) -conductance have been overlooked. In this context, we explore the specificity and the cellular toxicity of both inhibitors in CFTR-expressing and non-CFTR-expressing cells. EXPERIMENTAL APPROACH: Using patch-clamp technique, we tested the effects of CFTRinh -172 and GlyH-101 inhibitors on three distinct types of Cl(-) currents: the CFTR-like conductance, the volume-sensitive outwardly rectifying Cl(-) conductance (VSORC) and finally the Ca(2+) -dependent Cl(-) conductance (CaCC). We also explored the effect of both inhibitors on cell viability using live/dead and cell proliferation assays in two different cell lines. KEY RESULTS: We confirmed that these two compounds were potent inhibitors of the CFTR-mediated Cl(-) conductance. However,GlyH-101 also inhibited the VSORC conductance and the CaCC at concentrations used to inhibit CFTR. The CFTRinh -172 did not affect the CaCC but did inhibit the VSORC, at concentrations higher than 5 microM. Neither inhibitor (20 microM; 24 h exposure) affected cell viability, but both were cytotoxic at higher concentrations. CONCLUSIONS AND IMPLICATIONS: Both inhibitors affected Cl(-) conductances apart from CFTR. Our results provided insights into their use in mouse models.
Divergent CFTR orthologs respond differently to the channel inhibitors CFTRinh-172, glibenclamide, and GlyH-101.[Pubmed:21940661]
Am J Physiol Cell Physiol. 2012 Jan 1;302(1):C67-76.
Comparison of diverse orthologs is a powerful tool to study the structure and function of channel proteins. We investigated the response of human, killifish, pig, and shark cystic fibrosis transmembrane conductance regulator (CFTR) to specific inhibitors of the channel: CFTR(inh)-172, glibenclamide, and GlyH-101. In three systems, including organ perfusion of the shark rectal gland, primary cultures of shark rectal gland tubules, and expression studies of each ortholog in cRNA microinjected Xenopus laevis oocytes, we observed fundamental differences in the sensitivity to inhibition by these channel blockers. In organ perfusion studies, shark CFTR was insensitive to inhibition by CFTR(inh)-172. This insensitivity was also seen in short-circuit current experiments with cultured rectal gland tubular epithelial cells (maximum inhibition 4 +/- 1.3%). In oocyte expression studies, shark CFTR was again insensitive to CFTR(inh)-172 (maximum inhibition 10.3 +/- 2.5% at 25 muM), pig CFTR was insensitive to glibenclamide (maximum inhibition 18.4 +/- 4.4% at 250 muM), and all orthologs were sensitive to GlyH-101. The amino acid residues considered responsible by previous site-directed mutagenesis for binding of the three inhibitors are conserved in the four CFTR isoforms studied. These experiments demonstrate a profound difference in the sensitivity of different orthologs of CFTR proteins to inhibition by CFTR blockers that cannot be explained by mutagenesis of single amino acids. We believe that the potency of the inhibitors CFTR(inh)-172, glibenclamide, and GlyH-101 on the CFTR chloride channel protein is likely dictated by the local environment and the three-dimensional structure of additional residues that form the vestibules, the chloride pore, and regulatory regions of the channel.
Rescue of functional F508del cystic fibrosis transmembrane conductance regulator by vasoactive intestinal peptide in the human nasal epithelial cell line JME/CF15.[Pubmed:19584307]
J Pharmacol Exp Ther. 2009 Oct;331(1):2-13.
F508del is the most common cystic fibrosis-causing mutation that induces early degradation and poor trafficking of cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels to the apical membrane of epithelial cells. Our previous work in bronchial serous cells showed that vasoactive intestinal peptide (VIP) stimulation of the VPAC(1) receptor enhances CFTR-dependent chloride secretion by increasing its membrane insertion by a protein kinase C (PKC)-dependent pathway. In the present study, we investigated the effect of VIP on F508del-CFTR activity and membrane insertion in the human nasal epithelial cell line JME/CF15, which also expresses the VPAC(1) receptor. At reduced temperature (27 degrees C), which rescues F508del-CFTR trafficking, acute stimulation by VIP of rescued F508del-CFTR channels was protein kinase A (PKA)- and PKC-dependent. One hour of treatment with VIP strongly increased F508del-CFTR activity, with iodide efflux peaks three times higher than with untreated cells. At 37 degrees C, VIP-treated cells, but not untreated controls, showed significant iodide efflux peaks that were sensitive to the CFTR inhibitor 3-[(3-trifluoromethyl)phenyl]-5-[(4-carboxyphenyl)methylene]-2-thioxo-4-thiazolid inone (CFTR(inh)-172). Immunostaining, biotinylation assays, and Western blots confirmed a VIP-induced maturation and membrane insertion of F508del-CFTR at 37 degrees C. The corrector effect of VIP was abolished by the PKA inhibitor N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamidedihydrochloride (H89), whereas Galpha(s) stimulation by cholera toxin significantly increased F508del-CFTR trafficking. On the other hand, membrane localization, but not maturation, of F508del-CFTR was significantly reduced by the PKC inhibitor bisindolylmaleimide X and the G(i/o) protein inhibitor pertussis toxin. VIP treatment had no effect on intracellular calcium or proteasome activity. These results indicate that, in human nasal cells, VIP rescues trafficking and membrane insertion of functional F508del-CFTR channels at physiological temperature by stimulating both PKA- and PKC-dependent pathways.
Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease.[Pubmed:18385427]
J Am Soc Nephrol. 2008 Jul;19(7):1300-10.
Cyst expansion in polycystic kidney disease (PKD) involves progressive fluid accumulation, which is believed to require chloride transport by the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Herein is reported that small-molecule CFTR inhibitors of the thiazolidinone and glycine hydrazide classes slow cyst expansion in in vitro and in vivo models of PKD. More than 30 CFTR inhibitor analogs were screened in an MDCK cell model, and near-complete suppression of cyst growth was found by tetrazolo-CFTR(inh)-172, a tetrazolo-derived thiazolidinone, and Ph-GlyH-101, a phenyl-derived glycine hydrazide, without an effect on cell proliferation. These compounds also inhibited cyst number and growth by >80% in an embryonic kidney cyst model involving 4-d organ culture of embryonic day 13.5 mouse kidneys in 8-Br-cAMP-containing medium. Subcutaneous delivery of tetrazolo-CFTR(inh)-172 and Ph-GlyH-101 to neonatal, kidney-specific PKD1 knockout mice produced stable, therapeutic inhibitor concentrations of >3 microM in urine and kidney tissue. Treatment of mice for up to 7 d remarkably slowed kidney enlargement and cyst expansion and preserved renal function. These results implicate CFTR in renal cyst growth and suggest that CFTR inhibitors may hold therapeutic potential to reduce cyst growth in PKD.
Altered channel gating mechanism for CFTR inhibition by a high-affinity thiazolidinone blocker.[Pubmed:14759515]
FEBS Lett. 2004 Jan 30;558(1-3):52-6.
The thiazolidinone CFTR(inh)-172 was identified recently as a potent and selective blocker of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl(-) channel. Here, we characterized the CFTR(inh)-172 inhibition mechanism by patch-clamp and short-circuit analysis using cells stably expressing wild-type and mutant CFTRs. CFTR(inh)-172 did not alter CFTR unitary conductance (8 pS), but reduced open probability by >90% with K(i) approximately 0.6 microM. This effect was due to increased mean channel closed time without changing mean channel open time. Short-circuit current experiments indicated similar CFTR(inh)-172 inhibitory potency (K(i) approximately 0.5 microM) for inhibition of Cl(-) current in wild-type, G551D, and G1349D CFTR; however, K(i) was significantly reduced to 0.2 microM for DeltaF508 CFTR. Our studies provide evidence for CFTR inhibition by CFTR(inh)-172 by a mechanism involving altered CFTR gating.
Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion.[Pubmed:12464670]
J Clin Invest. 2002 Dec;110(11):1651-8.
Secretory diarrhea is the leading cause of infant death in developing countries and a major cause of morbidity in adults. The cystic fibrosis transmembrane conductance regulator (CFTR) protein is required for fluid secretion in the intestine and airways and, when defective, causes the lethal genetic disease cystic fibrosis. We screened 50,000 chemically diverse compounds for inhibition of cAMP/flavone-stimulated Cl(-) transport in epithelial cells expressing CFTR. Six CFTR inhibitors of the 2-thioxo-4-thiazolidinone chemical class were identified. The most potent compound discovered by screening of structural analogs, CFTR(inh)-172, reversibly inhibited CFTR short-circuit current in less than 2 minutes in a voltage-independent manner with K(I) approximately 300 nM. CFTR(inh)-172 was nontoxic at high concentrations in cell culture and mouse models. At concentrations fully inhibiting CFTR, CFTR(inh)-172 did not prevent elevation of cellular cAMP or inhibit non-CFTR Cl(-) channels, multidrug resistance protein-1 (MDR-1), ATP-sensitive K(+) channels, or a series of other transporters. A single intraperitoneal injection of CFTR(inh)-172 (250 micro g/kg) in mice reduced by more than 90% cholera toxin-induced fluid secretion in the small intestine over 6 hours. Thiazolidinone CFTR inhibitors may be useful in developing large-animal models of cystic fibrosis and in reducing intestinal fluid loss in cholera and other secretory diarrheas.


