TroxipideGastric cytoprotective agent CAS# 30751-05-4 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 30751-05-4 | SDF | Download SDF |
| PubChem ID | 5597 | Appearance | Powder |
| Formula | C15H22N2O4 | M.Wt | 294.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 9.09 mg/mL (30.88 mM; Need ultrasonic) H2O : 1 mg/mL (3.40 mM; ultrasonic and warming and heat to 80°C) | ||
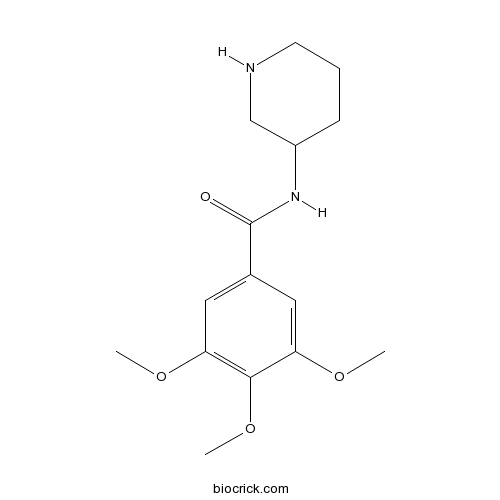
| Chemical Name | 3,4,5-trimethoxy-N-piperidin-3-ylbenzamide | ||
| SMILES | COC1=CC(=CC(=C1OC)OC)C(=O)NC2CCCNC2 | ||
| Standard InChIKey | YSIITVVESCNIPR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H22N2O4/c1-19-12-7-10(8-13(20-2)14(12)21-3)15(18)17-11-5-4-6-16-9-11/h7-8,11,16H,4-6,9H2,1-3H3,(H,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Troxipide is a novel gastro protective agent with antiulcer, anti-inflammatory and mucus secreting properties. |

Troxipide Dilution Calculator

Troxipide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3973 mL | 16.9866 mL | 33.9732 mL | 67.9463 mL | 84.9329 mL |
| 5 mM | 0.6795 mL | 3.3973 mL | 6.7946 mL | 13.5893 mL | 16.9866 mL |
| 10 mM | 0.3397 mL | 1.6987 mL | 3.3973 mL | 6.7946 mL | 8.4933 mL |
| 50 mM | 0.0679 mL | 0.3397 mL | 0.6795 mL | 1.3589 mL | 1.6987 mL |
| 100 mM | 0.034 mL | 0.1699 mL | 0.3397 mL | 0.6795 mL | 0.8493 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Troxipide is a novel systemic non-antisecretory gastric cytoprotective agent with anti-ulcer, anti-inflammatory and mucus secreting properties irrespective of pH of stomach or duodenum.
- 1,4-Bis[2-(4-methyl-5-phenyloxazolyl)]benzene
Catalog No.:BCC8425
CAS No.:3073-87-8
- WAY 170523
Catalog No.:BCC2380
CAS No.:307002-73-9
- CL 82198 hydrochloride
Catalog No.:BCC2372
CAS No.:307002-71-7
- GW-1100
Catalog No.:BCC1610
CAS No.:306974-70-9
- IBC 293
Catalog No.:BCC7376
CAS No.:306935-41-1
- TCID
Catalog No.:BCC4449
CAS No.:30675-13-9
- 5-Methoxy-7-hydroxycoumarin
Catalog No.:BCN3538
CAS No.:3067-10-5
- Cycloolivil
Catalog No.:BCN4081
CAS No.:3064-05-9
- (20S)-Protopanaxdiol
Catalog No.:BCN1254
CAS No.:30636-90-9
- AVE 0991 sodium salt
Catalog No.:BCC4222
CAS No.:306288-04-0
- 2,2-Diphenylglycine
Catalog No.:BCC8496
CAS No.:3060-50-2
- Spermine tetrahydrochloride
Catalog No.:BCC6864
CAS No.:306-67-2
- CFTRinh-172
Catalog No.:BCC4419
CAS No.:307510-92-5
- SMER 28
Catalog No.:BCC7908
CAS No.:307538-42-7
- STF 083010
Catalog No.:BCC6209
CAS No.:307543-71-1
- Crosemperine
Catalog No.:BCN2074
CAS No.:30785-56-9
- [Des-octanoyl]-Ghrelin (rat)
Catalog No.:BCC5953
CAS No.:307950-60-3
- beta-Anhydrouzarigenin
Catalog No.:BCN5222
CAS No.:3080-20-4
- L-Theanine
Catalog No.:BCN2571
CAS No.:3081-61-6
- AEBSF.HCl
Catalog No.:BCC1219
CAS No.:30827-99-7
- 4-Oxododecanedioic acid
Catalog No.:BCN5223
CAS No.:30828-09-2
- Spongouridine
Catalog No.:BCC9152
CAS No.:3083-77-0
- PM00104
Catalog No.:BCC4237
CAS No.:308359-57-1
- Aloesin
Catalog No.:BCN8437
CAS No.:30861-27-9
Troxipide, a novel antiulcer compound, has inhibitory effects on human neutrophil migration and activation induced by various stimulants.[Pubmed:11515628]
Dig Liver Dis. 2000 May;32(4):305-11.
BACKGROUND: Neutrophils are considered to be involved in the pathogenesis of Helicobacter pylori-associated gastroduodenal diseases on account of their potent biological functions as effector cells. Troxipide, a new antiulcer compound used for patients with gastric ulcer or gastritis, has been shown to inhibit migration and activation of guinea pig neutrophils, but little is known about the pharmacological effects on human neutrophils. AIMS: To study the effects of Troxipide on chemotactic migration and superoxide generation by human neutrophils. METHODS: The chemotactic response of neutrophils was determined in a multi-well chamber with a polycarbonate filter and the generation of O2- by neutrophils was measured using a chemiluminescence method. Concentrations of Troxipide in gastric mucosa were measured by high-performance liquid chromatography. RESULTS: Incubation of neutrophils with 10(-6) to 10(4) M Troxipide caused inhibition of recombinant interleukin-8-induced migration. These concentrations of Troxipide also inhibited superoxide generation by neutrophils stimulated by formyl-methionyl-leucyl-phenylalanine or platelet activating factor. These phenomena were not simply due to the direct cytotoxic effects since the above concentrations of Troxipide did not induce neutrophil apoptosis. The concentrations of Troxipide detected in the gastric mucosa after oral administration were in the range able to inhibit chemotactic migration and superoxide generation by neutrophils in vitro. CONCLUSION: These results suggest that Troxipide may exert its therapeutic effect in patients with gastric ulcer or gastritis by inhibiting inflammatory responses and mucosal injury mediated by neutrophils in gastric mucosa.
Design and evaluation of polyox and pluronic controlled gastroretentive delivery of troxipide.[Pubmed:25505995]
J Drug Deliv. 2014;2014:804616.
Objective. Objective of the present work was to develop site-specific gastroretentive drug delivery of Troxipide using polymers Pluronic F127 and Polyox 205 WSR. Troxipide is a novel gastroprotective agent with antiulcer, anti-inflammatory, and mucus secreting properties with elimination half-life of 7.4 hrs. Troxipide inhibits H. pylori-derived urease. It is mainly absorbed from stomach. Methods. 3(2) factorial design was applied to study the effect of independent variable. Effects of concentration of polymer on dependant variables as swelling index, hardness, and % drug release were studied. Pluronic F127 and Polyox 205 WSR were used as rate controlled polymer. Sodium bicarbonate and citric acid were used as effervescent-generating agent. Results. From the factorial batches, it was observed that formulation F5 (19% Pluronic F127 and 80% Polyox 205 WSR) showed optimum controlled drug release (98.60% +/- 1.82) for 10 hrs with ability to float >12 hrs. Optimized formulation characterized by FTIR and DSC studies confirmed no chemical interactions between drug and polymer. Gastroretention for 6 hrs for optimized formulations was confirmed by in vivo X-ray placebo study. Conclusion. Results demonstrated feasibility of Troxipide in the development of gastroretentive site-specific drug delivery.
Troxipide in the management of gastritis: a randomized comparative trial in general practice.[Pubmed:21127703]
Gastroenterol Res Pract. 2010;2010:758397.
Background. A trial of empirical acid-suppressive therapy is the usual practice for most patients with symptoms of gastritis in primary care. Aim. To assess the relative efficacy of Troxipide and Ranitidine in patients with endoscopic gastritis over a four-week period. Methods. In all, 142 patients were randomized to Troxipide (100 mg tid) or Ranitidine (150 mg bid) for a period of four weeks. The severity of the signs of endoscopic gastritis at baseline and week 4 using a four-point scale and the subjective symptom severity at baseline and week 2 & week 4 using a Visual analog scale (VAS) were documented. Results. Troxipide was found to be superior to Ranitidine for both, the complete resolution and improvement of endoscopic gastritis. Higher proportion of patients showed complete healing of erosions (88.14%), oozing (96.77%), and edema (93.88%) with Troxipide as compared to Ranitidine (P < .01). Patients receiving Troxipide also showed a greater improvement in the VAS scores for abdominal pain, bloating, and heartburn (P < .01). Both the drugs were found to be well tolerated. Conclusion. In patients with endoscopic gastritis, Troxipide, with its superior rate of improvement, resolution of signs, and subjective clinical symptoms, can be considered as an alternative to the commonly used antisecretory agents.
Preparation and pharmacokinetics study on gastro-floating sustained-release tablets of troxipide.[Pubmed:25190152]
Drug Dev Ind Pharm. 2015;41(9):1443-51.
The purpose of this research aimed at preparing gastro-floating sustained-release tablets of Troxipide and a further study on in vitro release and in vivo bioavailability. Under the circumstances of direct powder compression, the floating tablets were successfully prepared with HPMC as main matrix material, Carbopol as assistant matrix material, octadecanol as floating agent and sodium bicarbonate as foaming agent to float by gas-forming. The floating time and accumulative release amount as evaluation indexes were utilized to perform pre-experiment screening and single-factor test, respectively, while central composite design response surface method was applied for formulation optimization, followed by in vivo pharmacokinetic study in beagles after oral administration for floating tablets and commercial tablets used as the control. The results indicated that the floating sustained-release tablets held a better capability for floating and drug release and more satisfactory pharmacokinetic parameters, such as a lower Cmax, a prolonged Tmax, but an equivalent bioavailability calculated by AUC0-24 compared to commercial tablets. So a conclusion was finally drawn that the floating sustained-release tablets possessing a good release property could be suitable for demands of design.


