STF 083010IRE1α endonuclease inhibitor CAS# 307543-71-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 307543-71-1 | SDF | Download SDF |
| PubChem ID | 6537512 | Appearance | Powder |
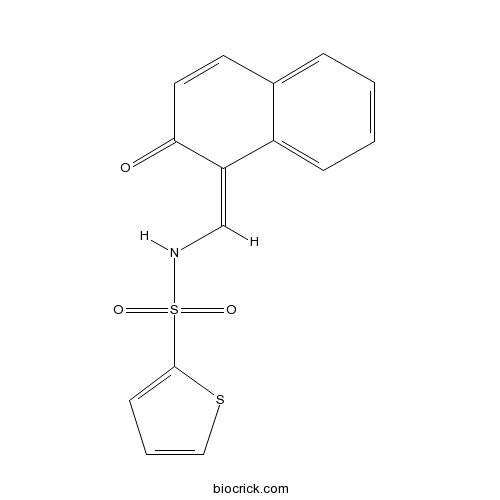
| Formula | C15H11NO3S2 | M.Wt | 317.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (315.08 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | N-[(Z)-(2-oxonaphthalen-1-ylidene)methyl]thiophene-2-sulfonamide | ||
| SMILES | C1=CC=C2C(=C1)C=CC(=O)C2=CNS(=O)(=O)C3=CC=CS3 | ||
| Standard InChIKey | AUXKGDNZANYAPX-RAXLEYEMSA-N | ||
| Standard InChI | InChI=1S/C15H11NO3S2/c17-14-8-7-11-4-1-2-5-12(11)13(14)10-16-21(18,19)15-6-3-9-20-15/h1-10,16H/b13-10- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of IRE1α endonuclease activity; blocks endogenous XBP1 mRNA splicing. Displays cytostatic and cytotoxic effects in CD138+ multiple myeloma (MM) cells in vitro; inhibits bortezomib-induced XBP1 activity in myeloma xenografts in vivo. Does not alter IRE1α kinase activity. |

STF 083010 Dilution Calculator

STF 083010 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1508 mL | 15.754 mL | 31.508 mL | 63.0159 mL | 78.7699 mL |
| 5 mM | 0.6302 mL | 3.1508 mL | 6.3016 mL | 12.6032 mL | 15.754 mL |
| 10 mM | 0.3151 mL | 1.5754 mL | 3.1508 mL | 6.3016 mL | 7.877 mL |
| 50 mM | 0.063 mL | 0.3151 mL | 0.6302 mL | 1.2603 mL | 1.5754 mL |
| 100 mM | 0.0315 mL | 0.1575 mL | 0.3151 mL | 0.6302 mL | 0.7877 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: N/A
STF 083010 is an inhibitor of IRE1α endonuclease activity, and does not alter IRE1α kinase.
In vitro: After endoplasmic reticulum stress both in vitro and in vivo, STF-083010 prevented Ire1 endonuclease activity without affecting its kinase activity. Treatment with STF-083010 exhibited significant antimyeloma activity in model human MM xenografts. Similarly, compared with other similarly isolated cell populations, STF-083010 was preferentially toxic to freshly isolated human CD138 MM cells. On basis of the identification of this novel Ire1 inhibitor, propose that the Ire1-XBP1 axis is a promising target for anticancer therapy (especially in the context of MM) [1].
In stark contrast, when apoptosis of tumor cells desired, therapeutic strategies attempted to stimulate ER stress with the aim of killing cancerous cells in the case of cancer treatment. For example, it enhanced the killing of these cancer cells that exposure of human multiple myeloma cells to STF083010, and that or treatment of a human pancreatic cancer cell line with bortezomib, [2].
In vivo: The small molecule STF083010, identified by a high-throughput screen of compounds affecting IRE1 activity, was capable of directly inhibiting the endonuclease function of IRE1 without affecting its kinase activity. After treatment of mice harboring subcutaneous xenografts led to tumor shrinkage and multiple myeloma cells harvested from cancer patients died following exposure to STF083010, the antimyeloma therapeutic potential of STF083010 was convincingly demonstrated [2].
Clinical trial: So far, no clinical study has been conducted.
References:
[1]. Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, Solow-Cordero DE, Bouley DM, Offner F, Niwa M, Koong AC. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011 Jan 27; 117 (4):1311-4.
[2]. Kraskiewicz H, FitzGerald U. InterfERing with endoplasmic reticulum stress. Trends Pharmacol Sci. 2012 Feb; 33 (2):53-63.
- SMER 28
Catalog No.:BCC7908
CAS No.:307538-42-7
- CFTRinh-172
Catalog No.:BCC4419
CAS No.:307510-92-5
- Troxipide
Catalog No.:BCC4744
CAS No.:30751-05-4
- 1,4-Bis[2-(4-methyl-5-phenyloxazolyl)]benzene
Catalog No.:BCC8425
CAS No.:3073-87-8
- WAY 170523
Catalog No.:BCC2380
CAS No.:307002-73-9
- CL 82198 hydrochloride
Catalog No.:BCC2372
CAS No.:307002-71-7
- GW-1100
Catalog No.:BCC1610
CAS No.:306974-70-9
- IBC 293
Catalog No.:BCC7376
CAS No.:306935-41-1
- TCID
Catalog No.:BCC4449
CAS No.:30675-13-9
- 5-Methoxy-7-hydroxycoumarin
Catalog No.:BCN3538
CAS No.:3067-10-5
- Cycloolivil
Catalog No.:BCN4081
CAS No.:3064-05-9
- (20S)-Protopanaxdiol
Catalog No.:BCN1254
CAS No.:30636-90-9
- Crosemperine
Catalog No.:BCN2074
CAS No.:30785-56-9
- [Des-octanoyl]-Ghrelin (rat)
Catalog No.:BCC5953
CAS No.:307950-60-3
- beta-Anhydrouzarigenin
Catalog No.:BCN5222
CAS No.:3080-20-4
- L-Theanine
Catalog No.:BCN2571
CAS No.:3081-61-6
- AEBSF.HCl
Catalog No.:BCC1219
CAS No.:30827-99-7
- 4-Oxododecanedioic acid
Catalog No.:BCN5223
CAS No.:30828-09-2
- Spongouridine
Catalog No.:BCC9152
CAS No.:3083-77-0
- PM00104
Catalog No.:BCC4237
CAS No.:308359-57-1
- Aloesin
Catalog No.:BCN8437
CAS No.:30861-27-9
- Kauran-18-Olc Acid,16,1719-Tnhydroxy-,(4A)
Catalog No.:BCC9235
CAS No.:308821-59-2
- 8-Epixanthatin
Catalog No.:BCN7782
CAS No.:30890-35-8
- 3β-Acetoxy-5α-androstan-17β-ol
Catalog No.:BCC8644
CAS No.:3090-70-8
A novel chemical, STF-083010, reverses tamoxifen-related drug resistance in breast cancer by inhibiting IRE1/XBP1.[Pubmed:26517687]
Oncotarget. 2015 Dec 1;6(38):40692-703.
Recent studies show that the unfolded protein response (UPR) within the endoplasmic reticulum is correlated with breast cancer drug resistance. In particular, human X-box binding protein-1(XBP1), a transcription factor which participates in UPR stress signaling, is reported to correlate with poor clinical responsiveness to tamoxifen. In this study, we develop a tamoxifen-resistant MCF-7 cell line by treating the cell line with low concentration of tamoxifen, and we find that XBP1 is indeed up-regulated at both the mRNA and protein levels compared to normal MCF-7 cells. STF-083010, a novel inhibitor which specifically blocks the XBP1 splicing, reestablishes tamoxifen sensitivity to resistant MCF-7 cells. Moreover, co-treatment with STF-083010 and tamoxifen can significantly delay breast cancer progression in a xenograft mammary tumor model. We next investigate the expression of XBP1s in over 170 breast cancer patients' samples and the results demonstrate that XBP1s expression level is highly correlated with overall survival in the ER+ subgroup, but not in the ER- subgroup, suggesting a potential therapeutic application of XBP1 inhibitors in ER+breast cancer treatment.
InterfERing with endoplasmic reticulum stress.[Pubmed:22112465]
Trends Pharmacol Sci. 2012 Feb;33(2):53-63.
Stress to the endoplasmic reticulum (ER) is a recognized factor in Alzheimer's and Parkinson's diseases, diabetes, heart disease, liver disorders and cancer. Thus, drugs that interfere with ER stress have wide therapeutic potential. Here we review the effects of drugs on three arms of ER stress: the protein kinase RNA-activated (PKR)-like ER kinase (PERK) arm, the activated transcription factor 6 (ATF6) arm and the inositol-requiring enzyme 1 (IRE1) arm. Drugs fall into five groups: (i) compounds directly binding to ER stress molecules; (ii) chemical chaperones; (iii) inhibitors of protein degradation; (iv) antioxidants; (v) drugs affecting calcium signaling. Treatments are generally inhibitory and lead to increased viability, except when applied to cancer cells. A focus on interfering with the ATF6 arm is required, and more in vivo testing of these compounds concurrently across all three arms is needed if the full importance of ER stress to human disease is to be realized.
Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma.[Pubmed:21081713]
Blood. 2011 Jan 27;117(4):1311-4.
Activation of the adaptive Ire1-XBP1 pathway has been identified in many solid tumors and hematologic malignancies, including multiple myeloma (MM). Here, we report the identification of STF-083010, a novel small-molecule inhibitor of Ire1. STF-083010 inhibited Ire1 endonuclease activity, without affecting its kinase activity, after endoplasmic reticulum stress both in vitro and in vivo. Treatment with STF-083010 showed significant antimyeloma activity in model human MM xenografts. Similarly, STF-083010 was preferentially toxic to freshly isolated human CD138(+) MM cells compared with other similarly isolated cell populations. The identification of this novel Ire1 inhibitor supports the hypothesis that the Ire1-XBP1 axis is a promising target for anticancer therapy, especially in the context of MM.


